the compression factor one mole of a vander waals gas 0 C and 100

By A Mystery Man Writer
Click here:point_up_2:to get an answer to your question :writing_hand:the compression factor for one mole of a vander waals gas at 0 c and
Click here👆to get an answer to your question ✍️ The compression factor one mole of a vander waals gas 0 C and 100 atm pressure is found to be 0-5

For one mole of a Vander Waals gas when b = 0 and T = 300 K, the PV vs 1/V plot is shown below. The value of the van der Waals


Bengali] The compressibility factor (Z) of one mole of a van der Waal

The compression factor (compressibility factor) for 1 mol of a van der

123. The compra compressibility factor one mole of a der Waals gas 0º C and 100 atm pressure found to be 0.5 Assume that the volume of es molecule is negligible. The
How to calculate the values of critical pressure and temperature for a given gas (Van der Waals equation) - Quora

Lecture 4-Real-Gases, PDF, Gases

123. The compra compressibility factor one mole of a der Waals gas 0º C and 100 atm pressure found to be 0.5 Assume that the volume of es molecule is negligible. The

Physical Chemistry The Compression Factor (Z) [w/1 example]
- Show that the van der Waals equation leads to values of Z <

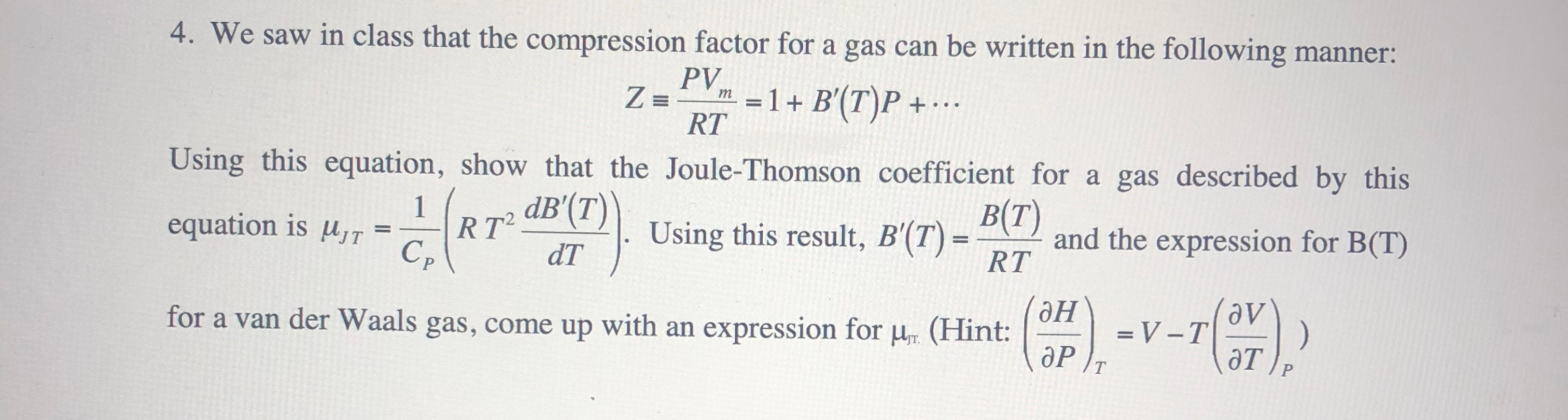
- Solved Z = 4. We saw in class that the compression factor
- Telugu] What is compressiblity factor?

- Find the isothermal compressibility `x` of a Van der Walls gas as a function of volume

- 53 pts!! The function f(x)= 7^x+1 is transformed to function g through a horizontal compression by a factor

- AIRROBO Dog Hair Vacuum & Dog Grooming Kit, 12000Pa

- Moda anti-idade: Melhores estilos de calça jeans para senhoras

- SUPER Nike red-label vintage AthDpt slicker / track pants - womens S

- Waterproof Shoe Covers Anti Slip PVC Rain Shoe Cover for Snow 33-34

- Korpiklaani & Týr: final de semana no Brasil – Portal Metal Revolution
