The compression factor (compressibility factor) for one mole of a van der Waals' gas - Sarthaks eConnect
By A Mystery Man Writer
The compression factor (compressibility factor) for one mole of a van der Waals
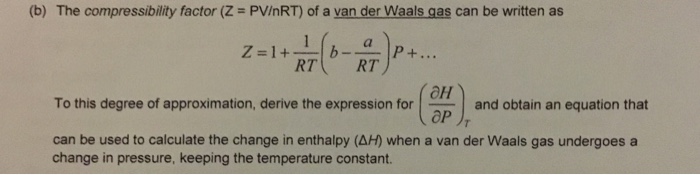
Solved (b) The compressibility factor (Z - PV/nRT) of a van
The compression factor (compressibility factor) for one mole of a van der Waals' gas - Sarthaks eConnect
For one mole real gas, the correct value of Z at point P using following graph is - Sarthaks eConnect

States Of Matter Notes: Class 11, JEE, NEET, AIIMS

The compressibility factor (Z) of one mole of a van der Waals' gas of negligible 'a' value is:1dfrac{bp}{RT}1+dfrac{bp}{RT}1-dfrac{bp}{RT}

Explain how the compression factor varies with pressure and

The compression factor (compressibility factor) one mole of a van der Waals' gas 0°C and 100 atm pressure is found to be 0.5. Assuming that the volume of a gas molecule is negligible, calculate the van der Waals' constant 'a' Domeik
The value of compression factor at the critical state of a vander waals gas is

The compression factor (compressibility factor) one mole of a van der Waals' gas 0°C and 100 atm pressure is found to be 0.5. Assuming that the volume of a gas molecule is negligible

Welcome to Chem Zipper.com: The compressibility factor for 1 mole of a van der Waals gas at 0oC and 100 atm pressure is found to be 0.5. Assuming that the volume of
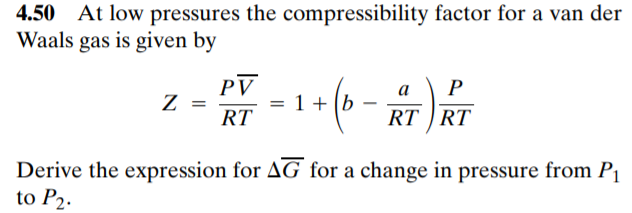
Solved 4.50 At low pressures the compressibility factor for
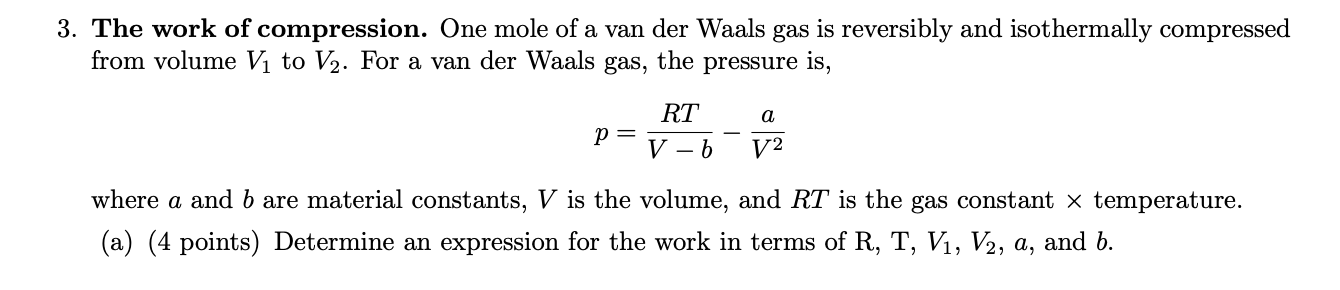
Solved 3. The work of compression. One mole of a van der
- Compressibility Factor of Gas, Overview, Equation & Chart - Lesson

- For H(2) gas, the compressibility factor,Z = PV //n RT is

- At high pressure, the compressibility factor 'Z' is equal toa)unityb) c) d)ZeroCorrect answer is option 'C'. Can you explain this answer? - EduRev NEET Question

- PPT - The Ideal Gas PowerPoint Presentation, free download - ID:6789672

- Compressibility - a basic concept in Fluid Mechanics

- Bipartisan group of senators unveil bill targeting TikTok, other foreign tech companies - CBS News

- Now Trending: Athletic Style Mens Athletic Fashion,, 54% OFF

- LELEBEAR Goldies Bra - Ultimate Lift Stretch Bra, 5D Nigeria

- See New David's Bridal Wedding Dresses for 2020 & 2021
- Bring It on' Director Talks 'Ideas' for a Sequel With Original Cast
