Sunday, Sept 29 2024
Establishing expiry date for clinical diagnostic reagents

By A Mystery Man Writer
Product shelf life is an essential product performance requirement that, along with other design requirements, is used to determine the safety and efficacy of a clinical diagnostic

Diagnostics and intellectual property - FIND

UNE EN ISO 23640:2015 In Vitro Diagnostic Medical Devices, 41% OFF

Implementing a resource management program for accreditation
:max_bytes(150000):strip_icc()/hlt-clinical-strength-deodorant-GROUPSHOT_tstaples_219-f2722289daa74184af4b93b9cc44933c.jpg)
The 9 Best Invisible Deodorants of 2024, Tested and Reviewed

Renji Brand CE Ivd Rapid Test Kit PCR Test Reagent - China PCR

How to Handle Lab Reagents After Their Expiration Date
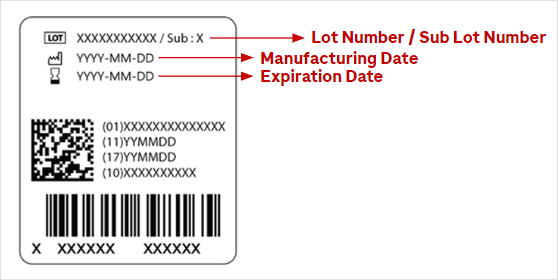
Pilot COVID-19 At-Home Test
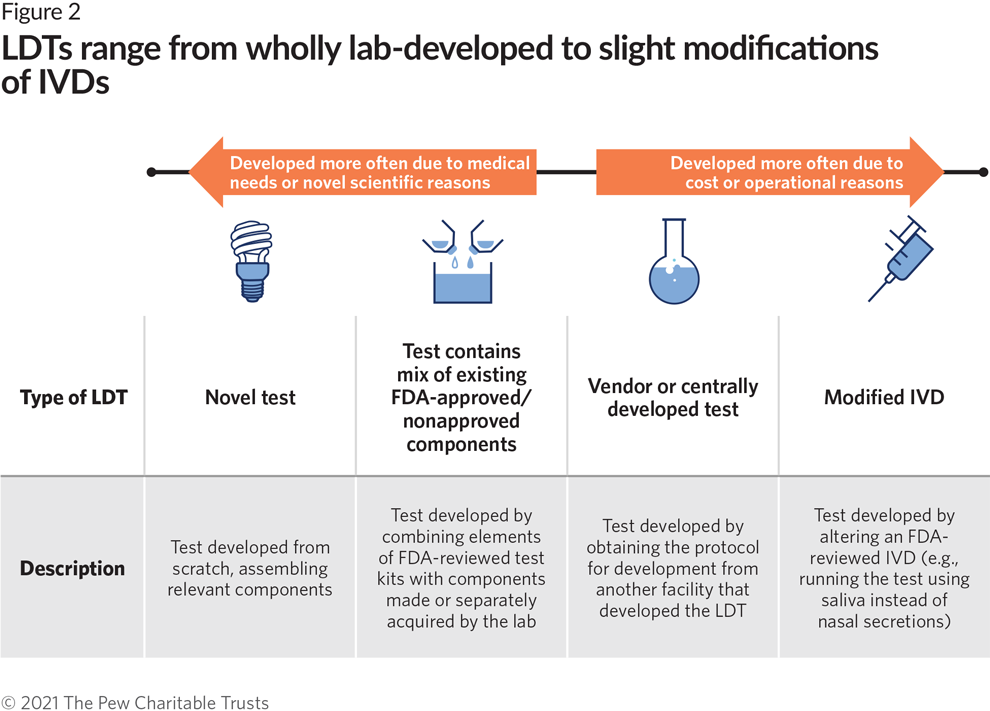
Diagnostic Tests Not Reviewed by FDA Present Growing Risks to

Mark Miller on LinkedIn: #diagnosticsispower
UNE EN ISO 23640:2015 In Vitro Diagnostic Medical Devices, 41% OFF
Related searches
- Here Are A Few Important Things To Know About Expiry Dates Of Food Products

- Medication Expiration Date Guidelines by FDA
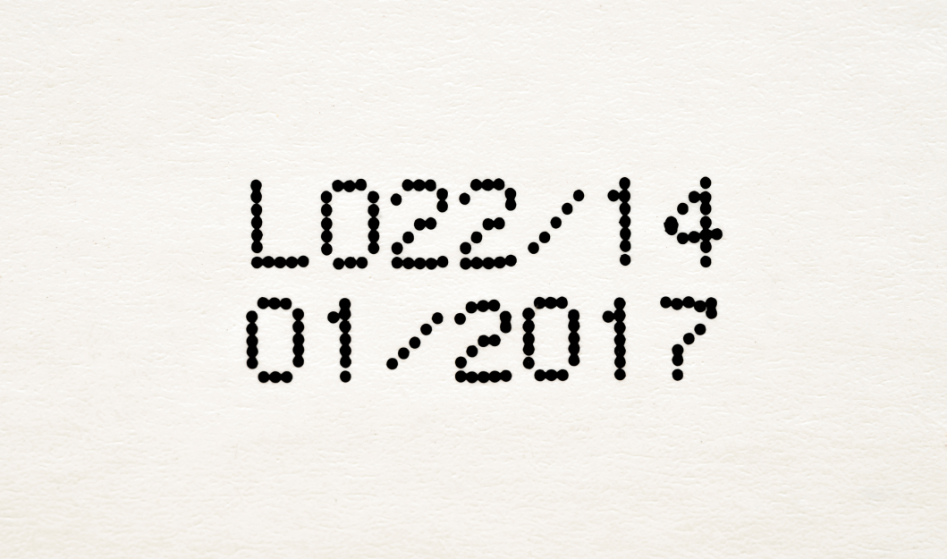
- How COVID Testing Helps Prep You for Respiratory Season
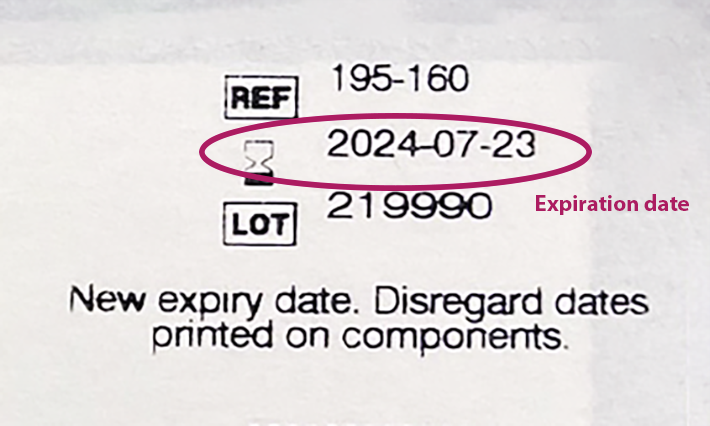
- How to read the product code on a Kit Kat candy bar for the expiration date - Quora
- When an Expiration Date Isn't Really an Expiration Date: Rapid At

Related searches
- Women Corset Crop Top Large L Gray Stretch Wide Straps Slim Square Neck

- Becca Modern Edge Sabrina Underwire Halter Bikini Top & Reviews

- Wilder 1.75 Cobra Belt with Integrated D-Ring

- Chicago's Top Tummy Tuck Recovery Tips - The Geldner Center

- ChongErfei 2 In 1 Postpartum Belly Band - Recovery Belly/Pelvis Belt Black Support Postpartum Belly Band

©2016-2024, doctommy.com, Inc. or its affiliates