Welcome to Chem Zipper.com: The compressibility factor for 1 mole of a van der Waals gas at 0oC and 100 atm pressure is found to be 0.5. Assuming that the volume of

By A Mystery Man Writer

The compressibility factor 1 mole of vanderwaal gas 0^{o}C, and 100 atm pressure is found to be 0.5, then calculate the vander Waals constant a. Assuming: that the volume of gas molecule

the compression factor one mole of a vander waals gas 0 C and 100 atm pressure is found to be 0.5

Solved APPENDIX Problem 1: Molar Volume and Compressibility

Welcome to Chem Zipper.com: A closed tank has two compartments A and B, both filled with oxygen (assumed to be ideal gas). The partition separating the two compartments is fixed and is
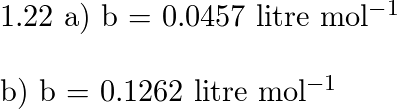
a) A certain gas obeys the van der Waals equation with $a =

Welcome to Chem Zipper.com: THE STATE OF MATTER
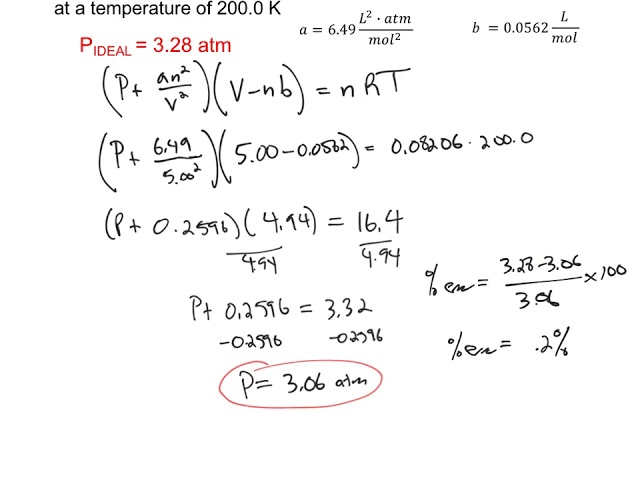
van der Waals example

Solved At high gas densities, the van der Waals equation der
The compression factor (compressibility factor) for one mole of a van der Waals' gas - Sarthaks eConnect

Solved We begin by showing that the compressibility factor
The compression factor (compressibility factor) for one mole of a van der Waals' gas - Sarthaks eConnect
The compressibility factor for one mol of a vanderwalls gas at 0 degree c and 100atm pressure is .5 then what will be the volume of 2 mols of this gas

Kirkwood–Buff-Derived Force Field for Peptides and Proteins: Applications of KBFF20

1148 questions with answers in GAS
How do Van der Waals constants a and b depend on temperature, pressure and volume? - Quora
- 1.7: Connecting the van der Waals and the viral equations: the Boyle temperature - Chemistry LibreTexts

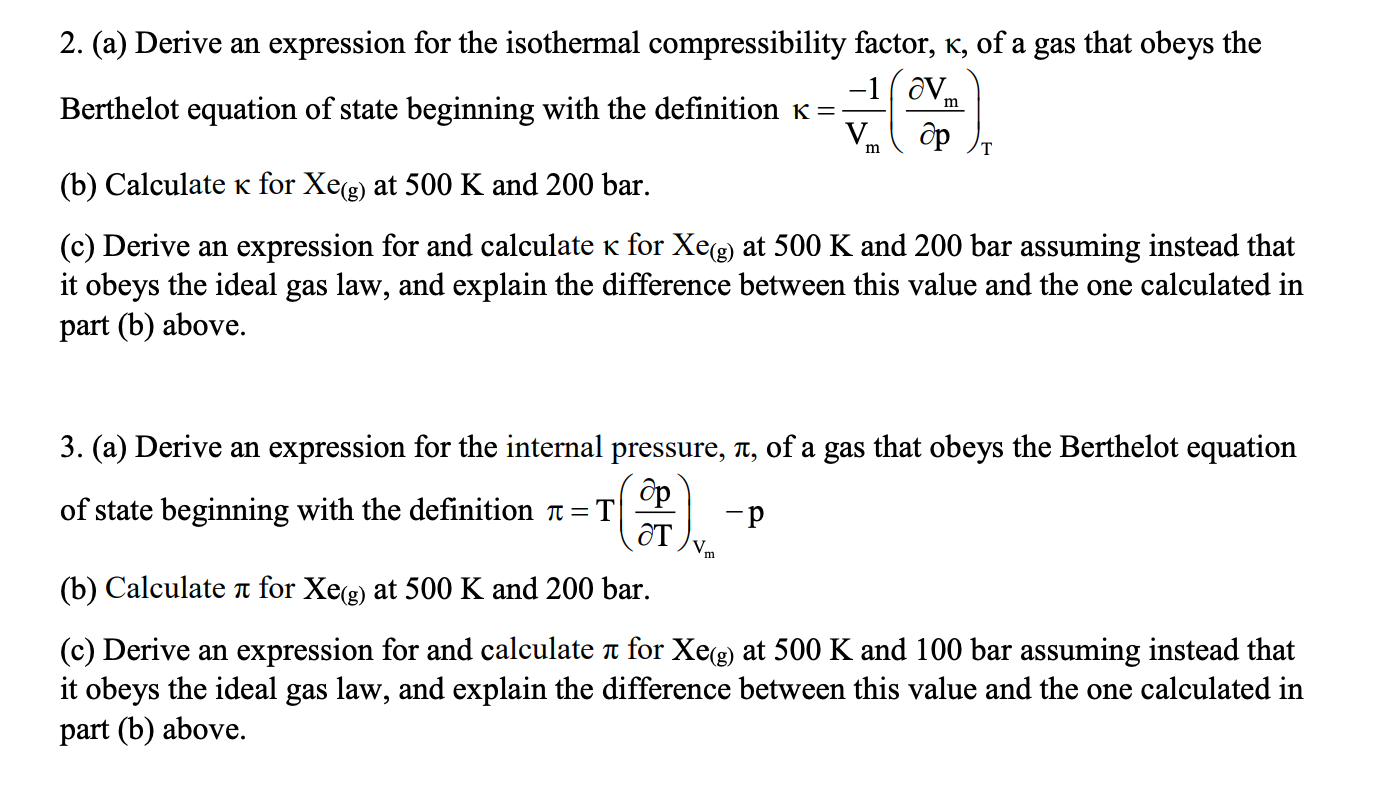
- Solved 2. (a) Derive an expression for the isothermal
- Solved We showed, for a van der Waals gas, that the
- Determine Compressibility of Gases

- Which of the following statements is/are correct? (a) all real

- Yoga Romper for Women Tummy Control Sculpting Sexy Thongs

- Issues of Westward Expansion: : Major Issues in American History

- Mens Cotton Green Jeans, 100% Cotton With 1% Elastane For Stretch

- Victoria's Secret, Pants & Jumpsuits, Victoria Secret Sport Black Full Length Mesh Panel Leggings Size Small

- ECHT, Pants & Jumpsuits, Echt Bum Scrunch Leggings