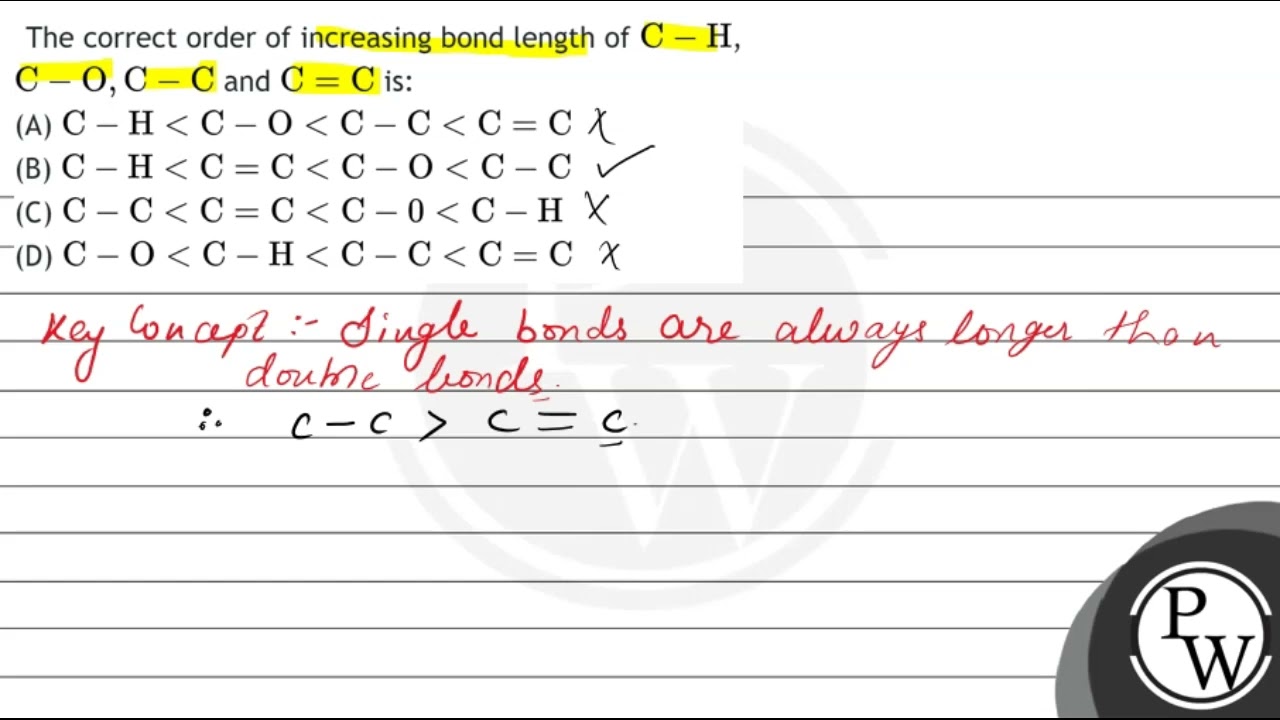
The correct order of increasing bond length of \( \mathrm{C
By A Mystery Man Writer
The correct order of increasing bond length of \( \mathrm{C}-\mathrm{H} \), \( \mathrm{C}-\mathrm{O}, \mathrm{C}-\mathrm{C} \) and \( \mathrm{C}=\mathrm{C} \

28. The correct order of increasing bond length of 3 C-H, C-, C-C and C = C is [AIPMT (Prelims)-2011] (1) C-H

The correct order of increasing bond length of C–H, C–O, C–C and C=C is - NEETLab

The correct order of increasing bond length of C - H ,C - O, C - C and
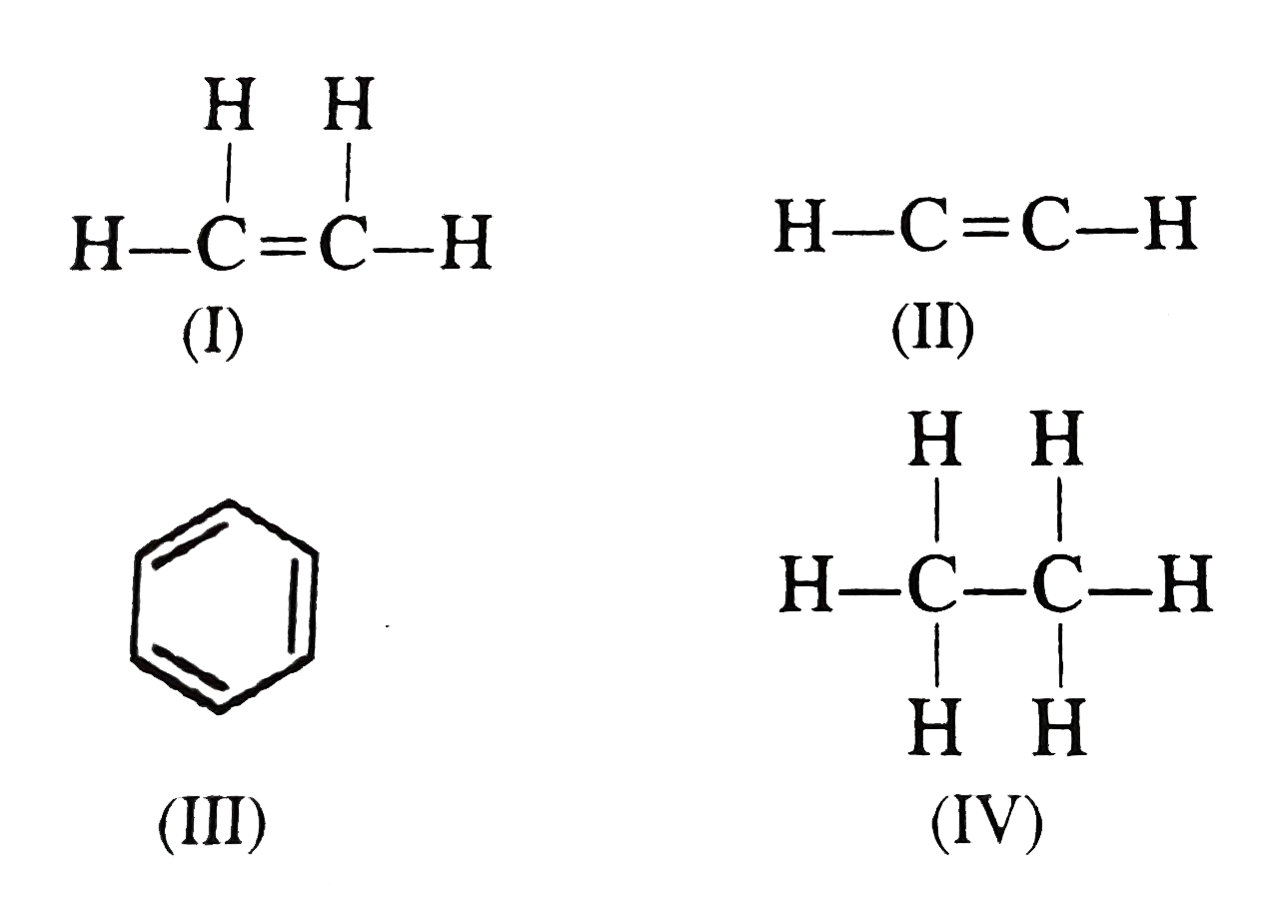
Decreasing order of C-C bond length is (I) C2H4 (II) C2H2 (III) C6 H6

OpenKIM · EAM Dynamo MendelevKramerOtt 2009 CuZr MO_600021860456_005 MO_600021860456 · Interatomic Potentials and Force Fields

In the Molecular Orbital diagram of SF6 ,find the sulfur orbitals which are non-bonding: px, py, pz dxy, dxz, dyz dx2 ??y2 , dz2 S . Explain your answer.
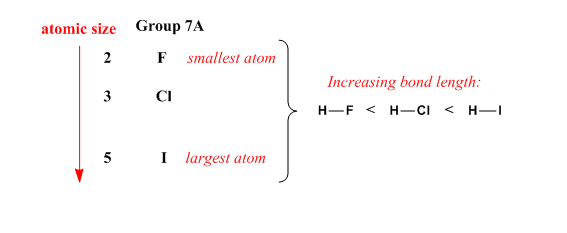
Using the periodic table only, arrange the members of each o

The correct order of increasing bond length of mathrm{C}-mathrm{H}, mathrm{C }-mathrm{O}, mathrm{C}-mathrm{C} and mathrm{C}=mathrm{C} is (1) mathrm{C}- mathrm{H}
- Harris Berson of Hall is doubled-teamed by Hartford defenders. Chase Hanawalt looks to provide an outlet for Berson - We-Ha

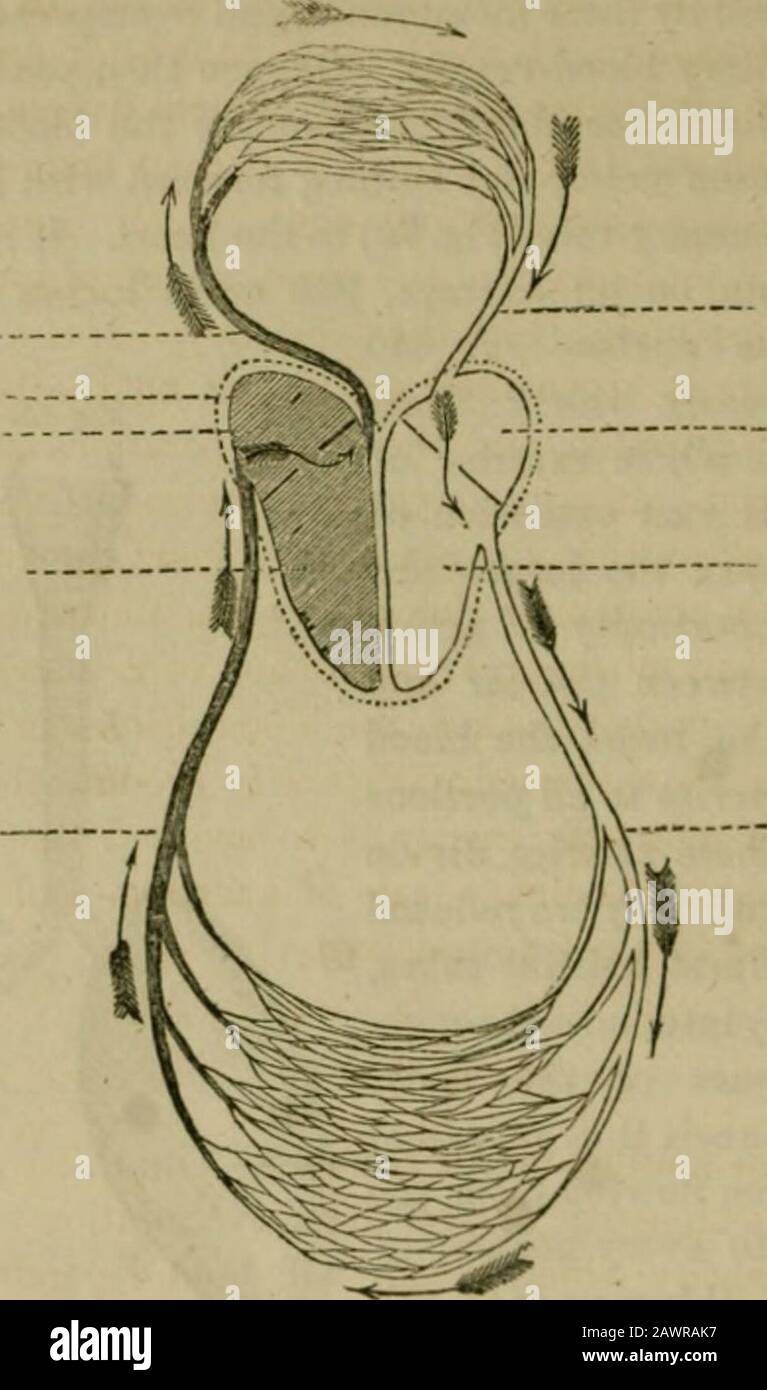
- The hand-book of household scienceA popular account of heat, light, air, aliment, and cleansing, in their scientific principles and domestic applicaions.. . d Human Lung.a the larynx; 6 windpipe; c c c
- Antique 1918 C.C.C. & St. L.R.R. Operating Rules Train

- DELIXI Small Circuit Breaker DZ47vP Air Switch C-Type D-Type Double-in Double-out 1P+N Household Short-Circuit Protection - AliExpress

- Square D QO290 2 Pole Circuit Breaker – SuperBreakers




