SOLVED: How much will the temperature of a cup (180 g) of coffee at 95 ^ C be reduced when a 45 g silver spoon (specific heat 0.24 J / g^∘C )

By A Mystery Man Writer
VIDEO ANSWER: We are asked how much the temperature of a cup of coffee it is at 95 degrees celsius and we put in there a 45 gram silver spoon. The heat capacity is 0.24 joules per gram, degrees c. So here's my mass here's, my c and my initial
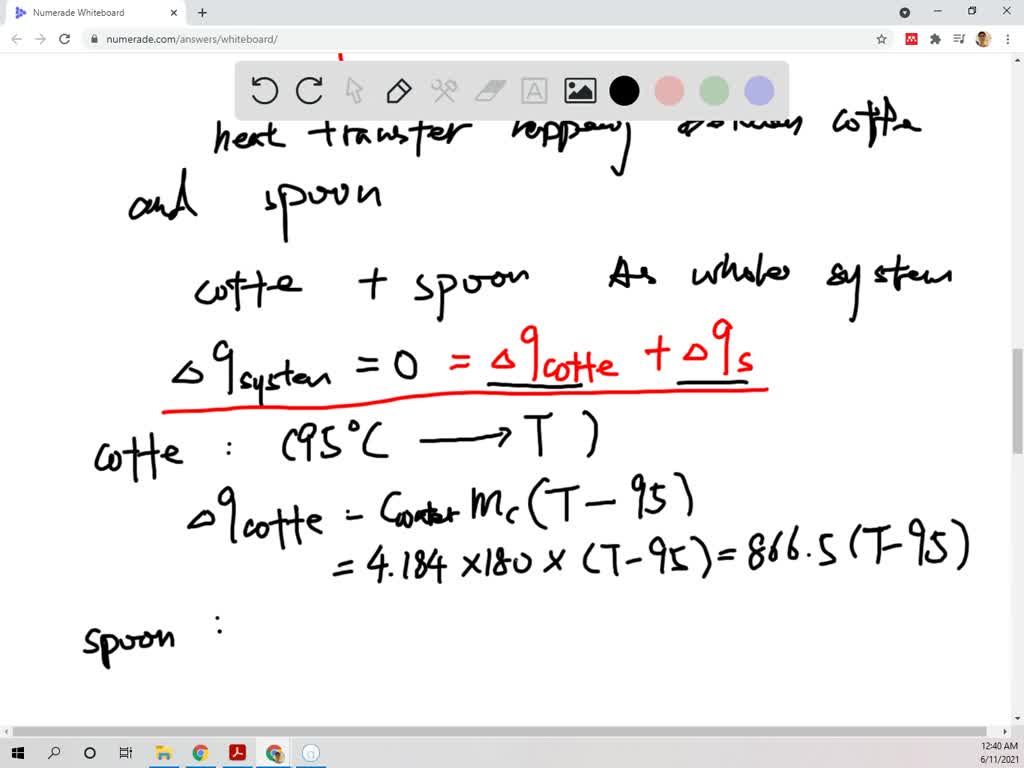
How much will the temperature of a cup (180 g) of coffee at 95 ^ C be reduced when a 45 g silver spoon (specific heat 0.24 J / g^∘C ) at 25^∘C is placed in the coffee and the two are allowed to reach the same temperature? Assume that the coffee has the same density and specific heat as water.
Numerade is a venture-backed, high-growth education technology startup based in Pasadena. We are singularly focused on creating exceptional video and interactive content experiences for education making the knowledge and skills of world class educators widely accessible and affordable to student audiences of all backgrounds. Our mission is to close the educational opportunity gap by unlocking and democratizing access to extraordinary educators and the content they have to offer.
⏩SOLVED:How much will the temperature of a cup (180 g) of coffee at…
Solved A 34.0 g silver spoon at 18.0∘C is placed in a cup of

5.19 How much will the temperature of a cup (180 g) of coffee at 95 °C be reduced when a 45 g

⏩SOLVED:How much will the temperature of a cup (180 g) of coffee at…
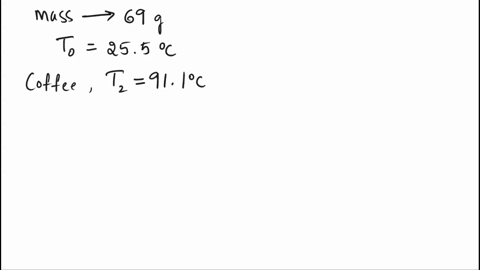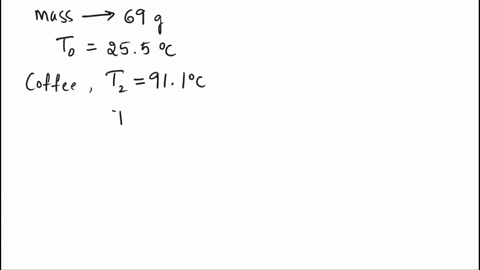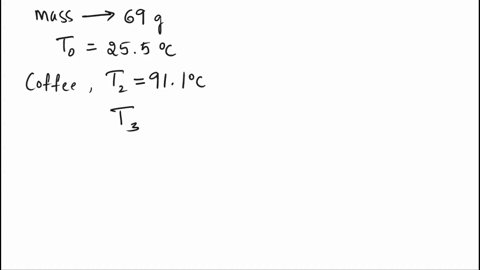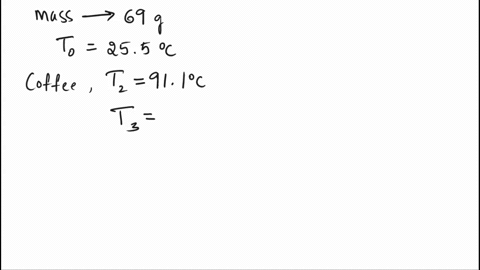
SOLVED: A 69.0 g silver spoon at 25.5°C is placed in a cup of coffee at 91.1°C. How much heat does the spoon absorb from the coffee to reach a temperature of

Solved 1. How much heat, in joules and in calories, must be

SOLVED: How much will the temperature of a cup (180 g) of coffee at 95 ^ C be reduced when a 45 g silver spoon (specific heat 0.24 J / g^∘C )

Calorimetry - Chemistry
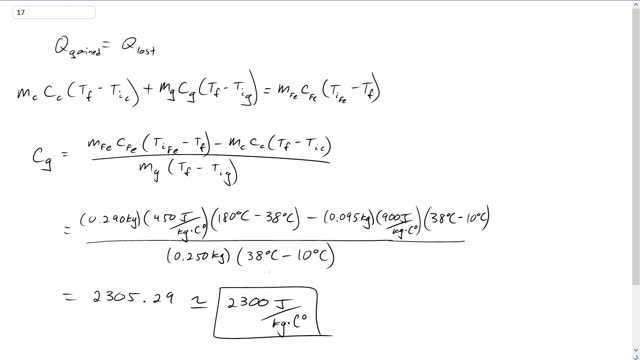
Giancoli 7th Edition, Chapter 14, Problem 17

SOLVED: please give soon assistance A 50.0 g silver spoon at 20.0 °C is placed in a cup of coffee at 90.0 °C. How much heat does the spoon absorb from the
A 110g sample of Copper (Copper=.385 j/g°C) is heated to 82.4°C and then placed in a insulated container of water (water=4.184 j/g°C) at 22.3°C.The final temperature of the water and copper is
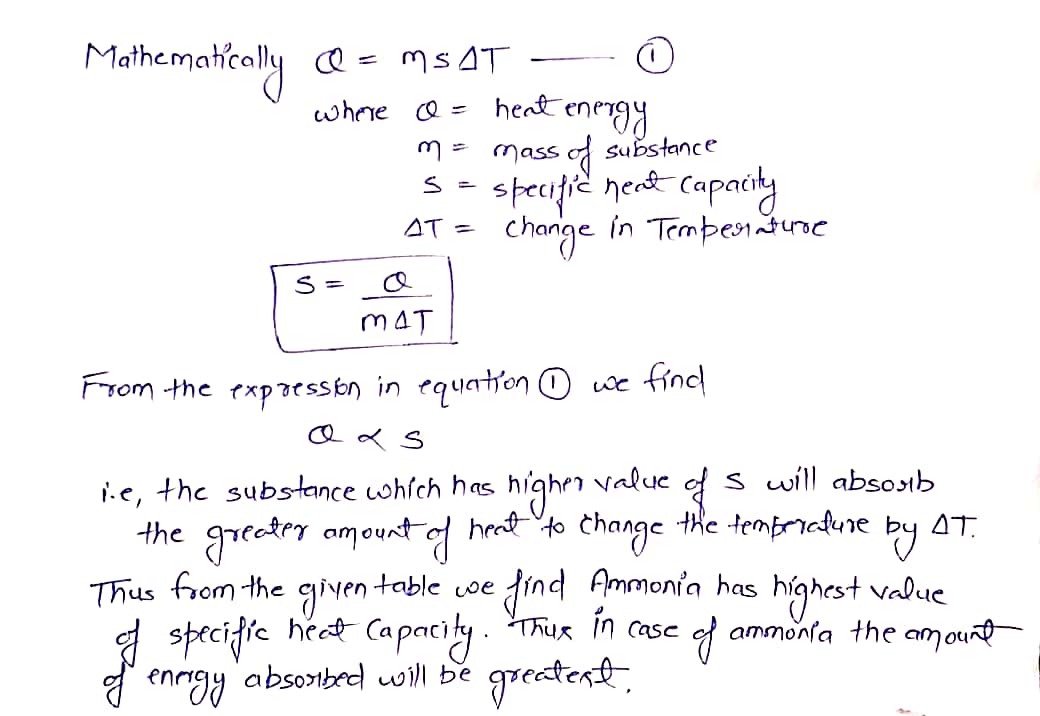
Answered: The specific heat capacities for…

SOLVED: A 50.2 g silver spoon at 16.1°C is placed in a cup of coffee at 94.0°C. A short time later, the spoon is at 87.5°C. If, during this same time, the

SOLVED: A 69.0 g silver spoon at 25.5°C is placed in a cup of coffee at 91.1°C. How much heat does the spoon absorb from the coffee to reach a temperature of
- New Senita Athletics Size XL Open Sides Tank Top Purple Crew Neck

- Brassiere demi Wacoal con copa para mujer

- Joy Lab Art Deco Print Yoga Leggings Gym Tights Pants

- Twinbirds Butter Scotch women Ankle Legging
- Menstrual period concept. Womens thighs with blood-stained panties. Feminine hygiene. Menstrual protection. Stock illustration. For website and article design, application and print. Stock Illustration






