The Cottrell Experiment and Diffusion Limitation 3/3

By A Mystery Man Writer
In this chapter the electrochemical double layer and its features are discussed. The electrochemical double layer acts as a capacitor and every change in the potential of the electrode will induce a capacitive charging current that is caused by physics not by a chemical reaction. This current decays exponentially.

Phase Transformation Lecture 3

Basic potential step and sweep methods

Deep Coupling Network For Multivariate Time Series Forecasting

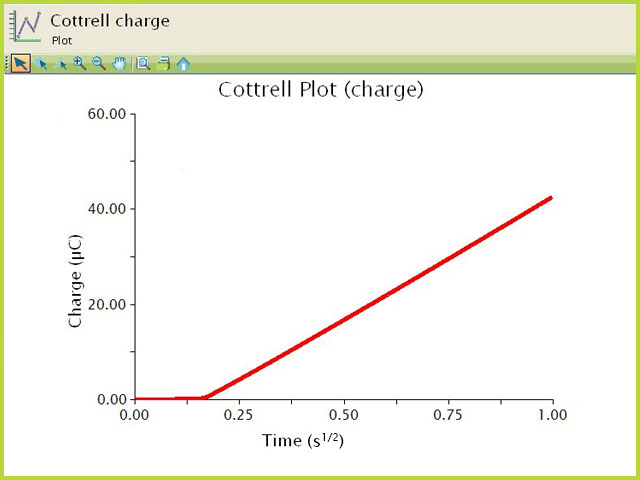
5 Mass transport (*diffusion, Fick's laws, Cottrell equation, Nernst diffusion layer)

Chemosensors, Free Full-Text

Electrochemistry with Stationary Disk and Ring−Disk Millielectrodes in Magnetic Fields

PDF) Comparison between Cottrell diffusion and moving boundary models for determination of the chemical diffusion coefficients in ion-insertion electrodes
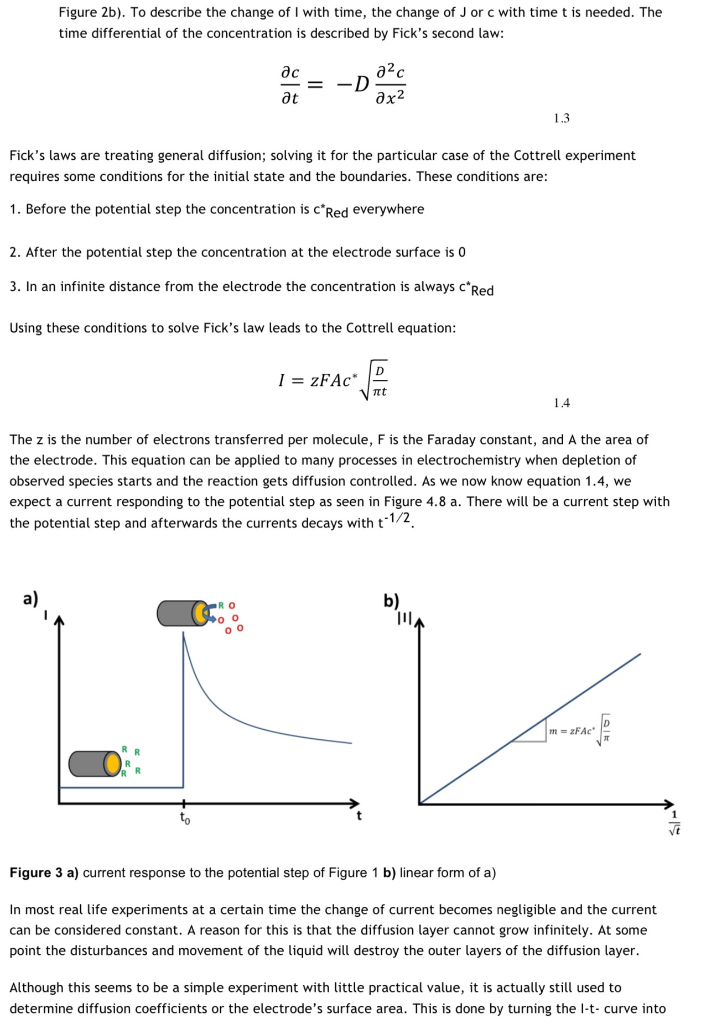
Figure 1.1: Cottrell experiment in KCl solution with
Chronoamperometry (CA) – Pine Research Instrumentation Store

The Cottrell Experiment and Diffusion Limitation 3/3
- FLKAYJM Shapewear for Women Tummy Control Body Shaper Under Dress Girdles Extra Firm Maidenform Middle Waisted Lingerie Panties Tummy Control Thigh Slimming Technology,5 Pack - Size M at Women's Clothing store

- Women's Shapewear Bodysuits Tummy Tuck Bodysuit With Bra Support Deep V Shapewear Bodysuit Corset

- Lead Free Brass Compression Fittings - 45 Degree Elbows - 1/4 Tube O.D. x 1/8 MIP

- 9 Honeymoon dress ideas for every couple – News9Live

- Womens Lingerie Sets Fashion Embroidery Lingerie Set Women Bras C D Cup Plus Size Underwear Set Black Transparent Bra And Panties Lace Bralette