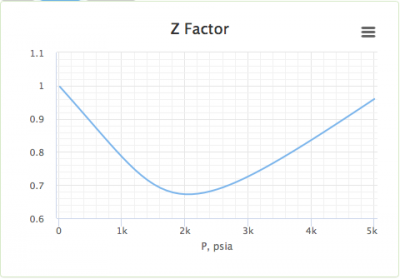
Compressibility factor Z = PV / nRT is plotted against pressure as shown below:What is the correct order for the liquefiability of the gases shown in the above graph? A. CO 2

By A Mystery Man Writer
Compressibility factor Z = PV / nRT is plotted against pressure as shown below:What is the correct order for the liquefiability of the gases shown in the above graph? A. CO 2
Compressibility factor Z - PV - nRT is plotted against pressure as shown below-What is the correct order for the liquefiability of the gases shown in the above graph- A- CO 2- CH 4- N 2- H 2B- H 2- CH 4- N 2- CO 2C- CH 4- H 2- N 2- CO 2D- H 2- N 2- CH 4- CO 2

3.2 Real gas and compressibility factor – Introduction to

Multi-scale simulation of wave propagation and liquefaction in a

PDF) Petroleum and natural gas production engineering

PV Compressibility factor Z= nRT is plotted against pressure : N

gaseous state

y factor Compressibility factor 2 V is plotted agalnst pressure RT

physical chemistry - Why do some gases have lower value of Z for a

PV Compressibility factor Z= nRT is plotted against pressure : N
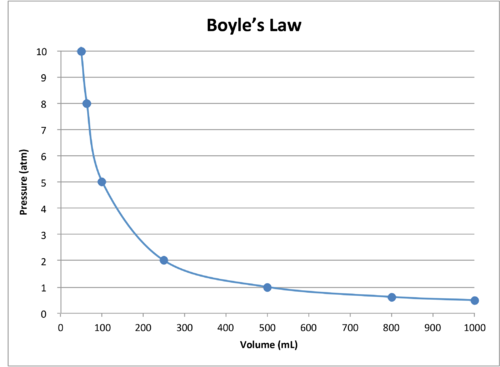
The Behavior of Gases Chemistry for Non-Majors
- Madamelle Strapless Bra - Luella, full lace detailing – madamelle

- World's sexiest volleyball star Kayla Simmons shows off incredible trick with her boobs leaving fans stunned

- Creating and Periodizing a Strength & Conditioning Program
- Nike Yoga Luxe Dri-FIT Women's Full-Zip Jacket, X-LARGE, Grey

- Missguided Womens 14 Dress Gown Green Tank Satin Zip Ruffle Hem Hi