200 g of a sample of limestone liberates 66 g of CO2 on heating
By A Mystery Man Writer
200 g of a sample of limestone liberates 66 g of CO2 on heating. The percentage purity of CaCO3 in the limestone is Options:a 95
200 g of a sample of limestone liberates 66 g of CO2 on heating- The percentage purity of CaCO3 in the limestone is Options-a- 95

Sustainability, Free Full-Text

PhEd-Some Basic Concepts of Chemistry-W.S, PDF, Mole (Unit)

400 g sample of limestone liberates 44 g carbon dioxide on heating. The p..
What is the mass of a 75% pure CaCO3 sample which on heating gives 2.2 g CO2 with 50% yield? - Quora
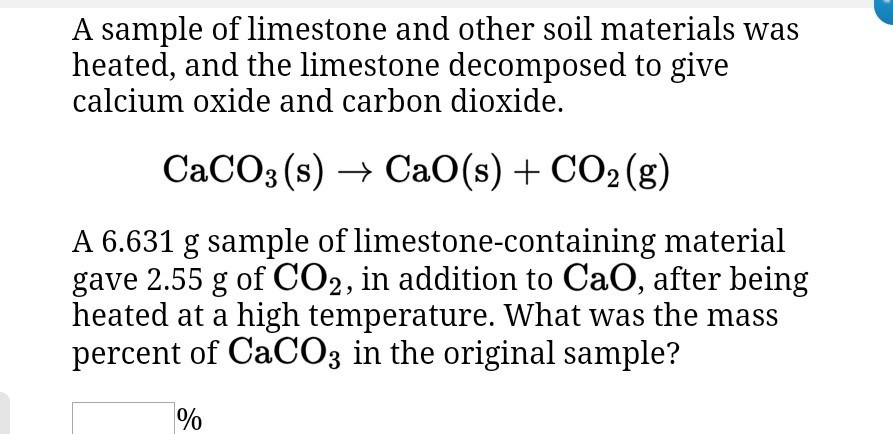
Solved A sample of limestone and other soil materials was

CHEMICAL REACTION AND EQUATIONS
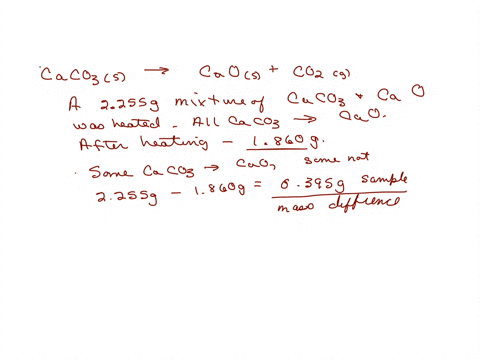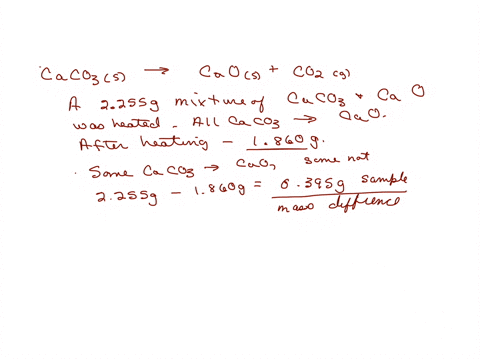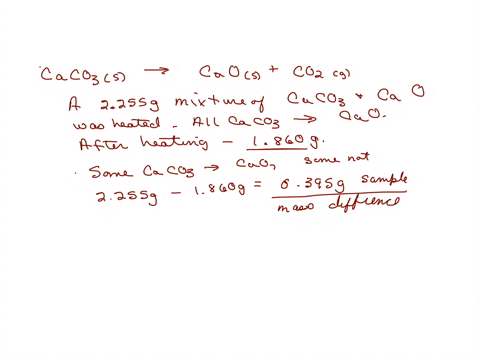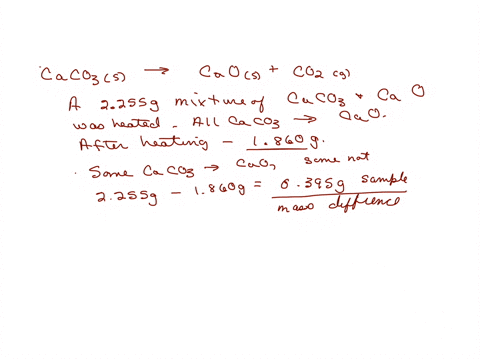
SOLVED: A sample of limestone and other soil materials was heated
qph.cf2.quoracdn.net/main-qimg-e6c92e9f07d768e12c6
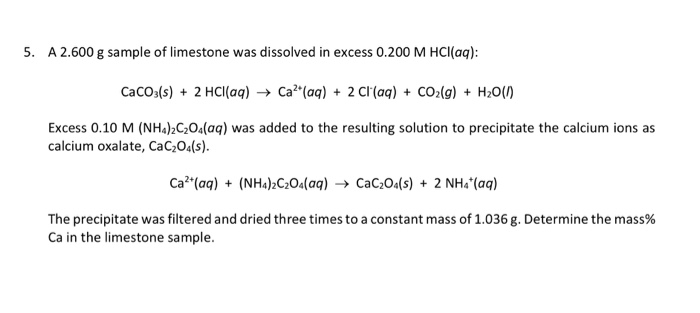
Solved A 2.600 g sample of limestone was dissolved in excess

SOLVED: Calcium carbonate (limestone) decomposes when heated: CaCO3 CaO CO2 When 20.0g of calcium carbonate are decomposed, 11.2g of calcium oxide (lime), CaO, are formed. Calculate the mass of calcium oxide formed
Isilted'-States Patent (,9, - University of Utah
qph.cf2.quoracdn.net/main-qimg-41dd277e7ab961913b6

MC, PDF, Phase (Matter)
- Justknits Womens & Girls Lingerie Underwear 1990s Vintage Sewing Patte

- Hidden Fence of Minnesota Invisible Dog Fence® Alternative

- Kydra Athletics - The Nitro Shorts — a true wardrobe
- Pokemon Complete Set - Japanese Radiant Collection

- Columbia River Resort Womens Capri Pants 12 Beige Silk Cotton Pocket 23 Inseam






