Saturday, Oct 05 2024
Compression of a gas due to external pressure and the

By A Mystery Man Writer

The work done in adiabatic compression of `2` mole of an ideal monoatomic gas by constant
Consider a gas cylinder containing 0.250 moles of an ideal gas in a volume of 6.00 L with [algebra]

The Ideal Monatomic Gas

/wp-content/uploads/2021/05/en
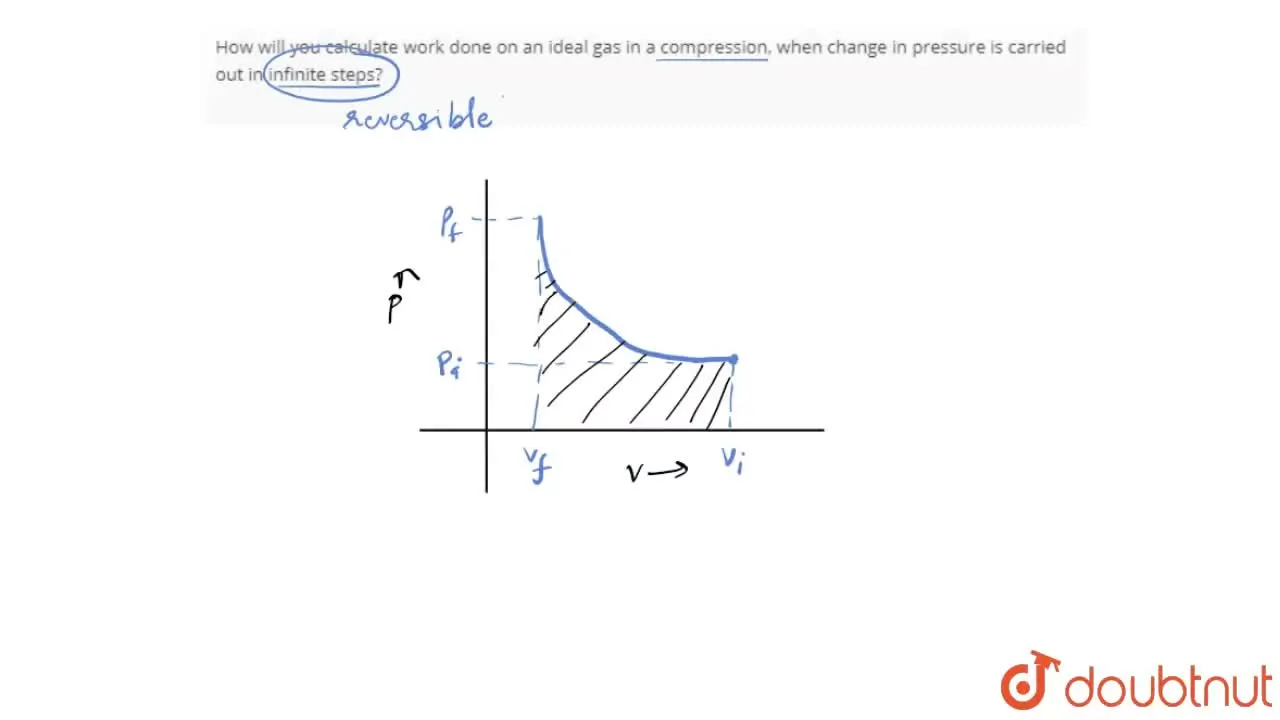
How will you calculate work done on an ideal gas in a compression, whe

12.2 First law of Thermodynamics: Thermal Energy and Work

2 mole of an ideal gas undergoes isothermal compression along three different paths

What happens to work when a gas expands against an external pressure? - Quora

External Pressure - Pressure Vessel Engineering
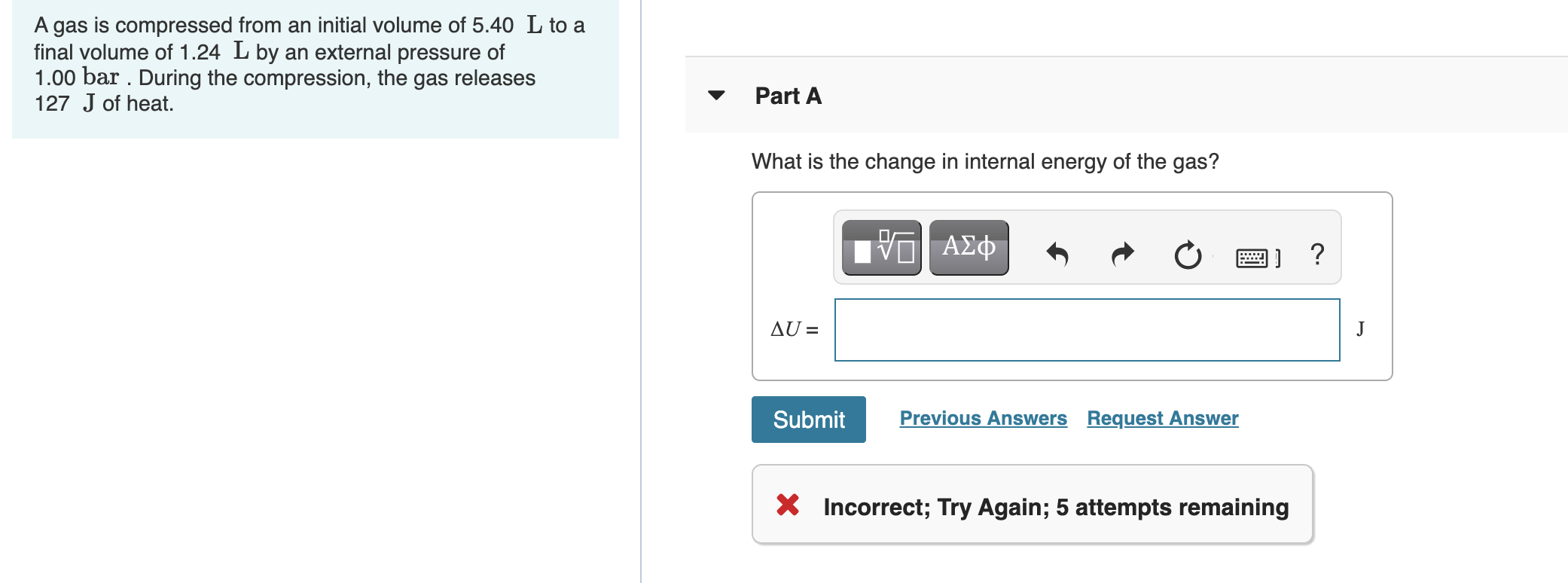
Solved A gas is compressed from an initial volume of 5.40 L

Compression of a gas due to external pressure and the corresponding
Related searches
Related searches
- Women Pack of 4 Seamless Lace Bra Comfort Stretchy Wirefree

- Premium Photo African american female in sportswear exercise pulling down wire cable on gym equipment in fitness

- Uotmiki Women's Lace Push Up Strapless Bra, Seamless Wirefree Non

- Do a Christmas Scavenger Hunt This Year — Thrifty Mommas Tips

- Ardyss Body Magic - Ardyss Body Magic is all you need if you want
©2016-2024, doctommy.com, Inc. or its affiliates
.jpg)



