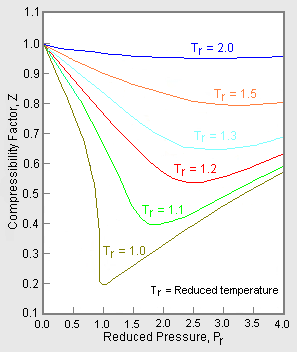
In the following compressibility factor Z vs pressure graph at 300

By A Mystery Man Writer
In the following compressibility factor Z vs pressure graph at 300 K, the compressibility of CH 4 at pressure
In the following compressibility factor Z vs pressure graph at 300 K- the compressibility of CH 4 at pressure -200 bar deviates from ideal behaviourA- The molar volume of CH 4 is less than its molar volume in the ideal stateB- The molar volume of CH 4 is same as that in its ideal stateC- Intermolecular interactions between CH 4 molecules decresasesD- The molar volume of CH 4 is more than its molar volume in the ideal state

In the following compressibility factor Z vs pressure graph at 300 K, the compressibility of CH 4 at pressure

Modus Operandi for Practice: A Comprehensive Study Package on Atomic Structure and the Gaseous State, PDF, Spectroscopy

Compressibility Charts McGraw-Hill Education - Access Engineering

Kvpy 2016 SB - SX Stream PCM, PDF, Electron

Energies, Free Full-Text
In the following compressibility factor Z vs pressure graph at 300 K the compressibility of CH4 at pressure < 200 bar deviates from ideal behaviour becauseA The molar volume of CH4 is

Gas Laws / Gases Behaviour, PDF, Gases

Kvpy 2016 SB - SX Stream PCM, PDF, Electron

Solved 20+ 15 10 9. Consider the gas compressibility factor

Modus Operandi for Practice: A Comprehensive Study Package on Atomic Structure and the Gaseous State, PDF, Spectroscopy
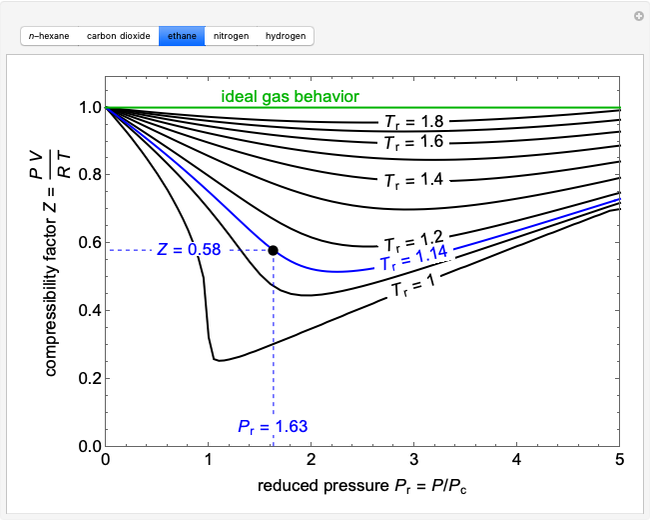
Compressibility Factor Charts - Wolfram Demonstrations Project