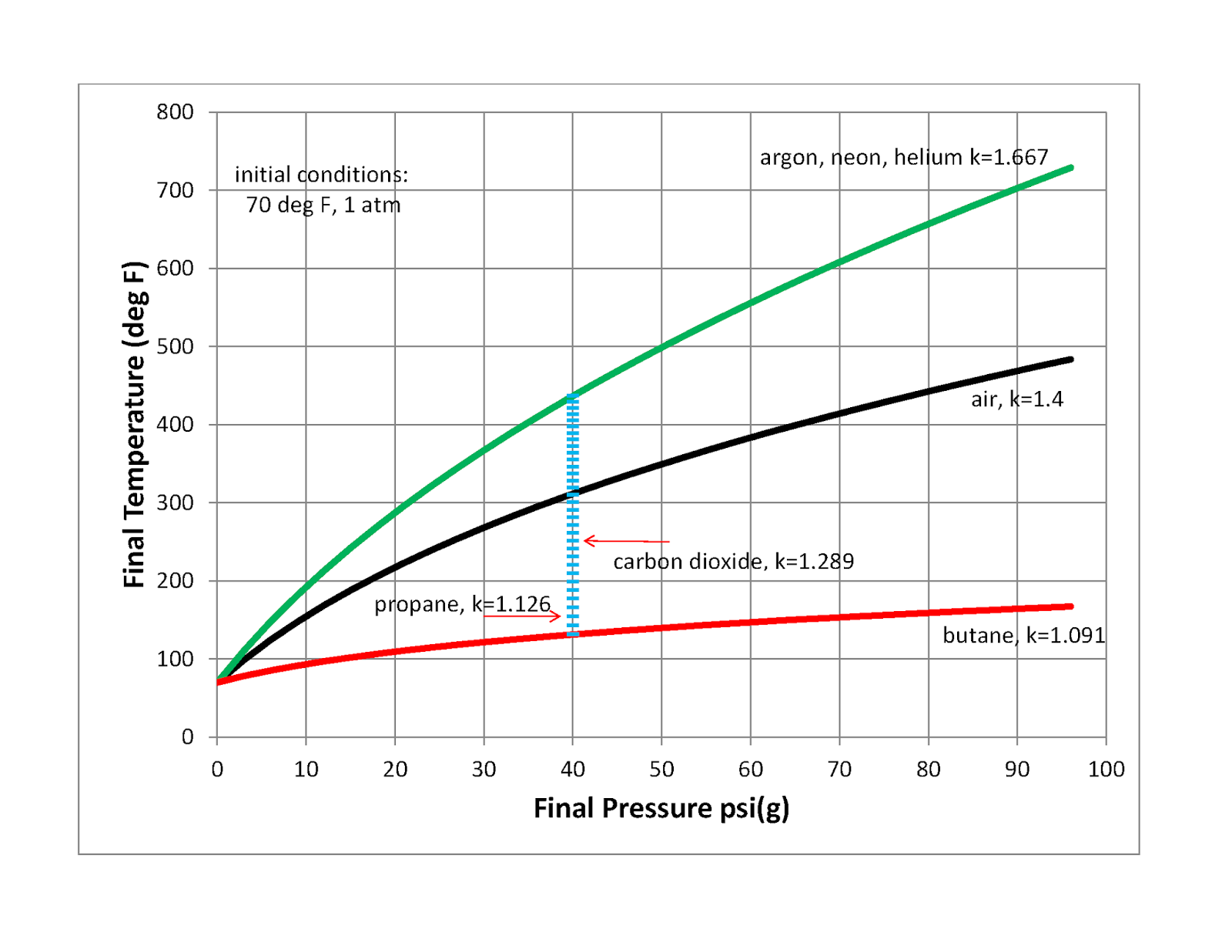
Why do pressure and temperature increase during the compression of

By A Mystery Man Writer
The energy added as work during the compression of a gas leads to an increase in pressure and temperature. Learn more about this in this article.
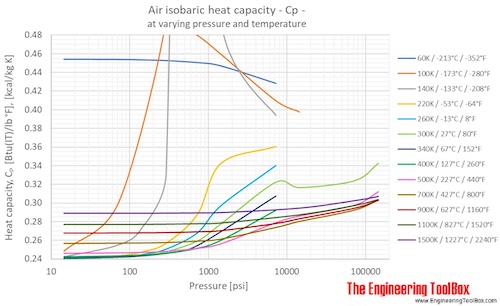
Air - Specific Heat vs. Pressure at Constant Temperature
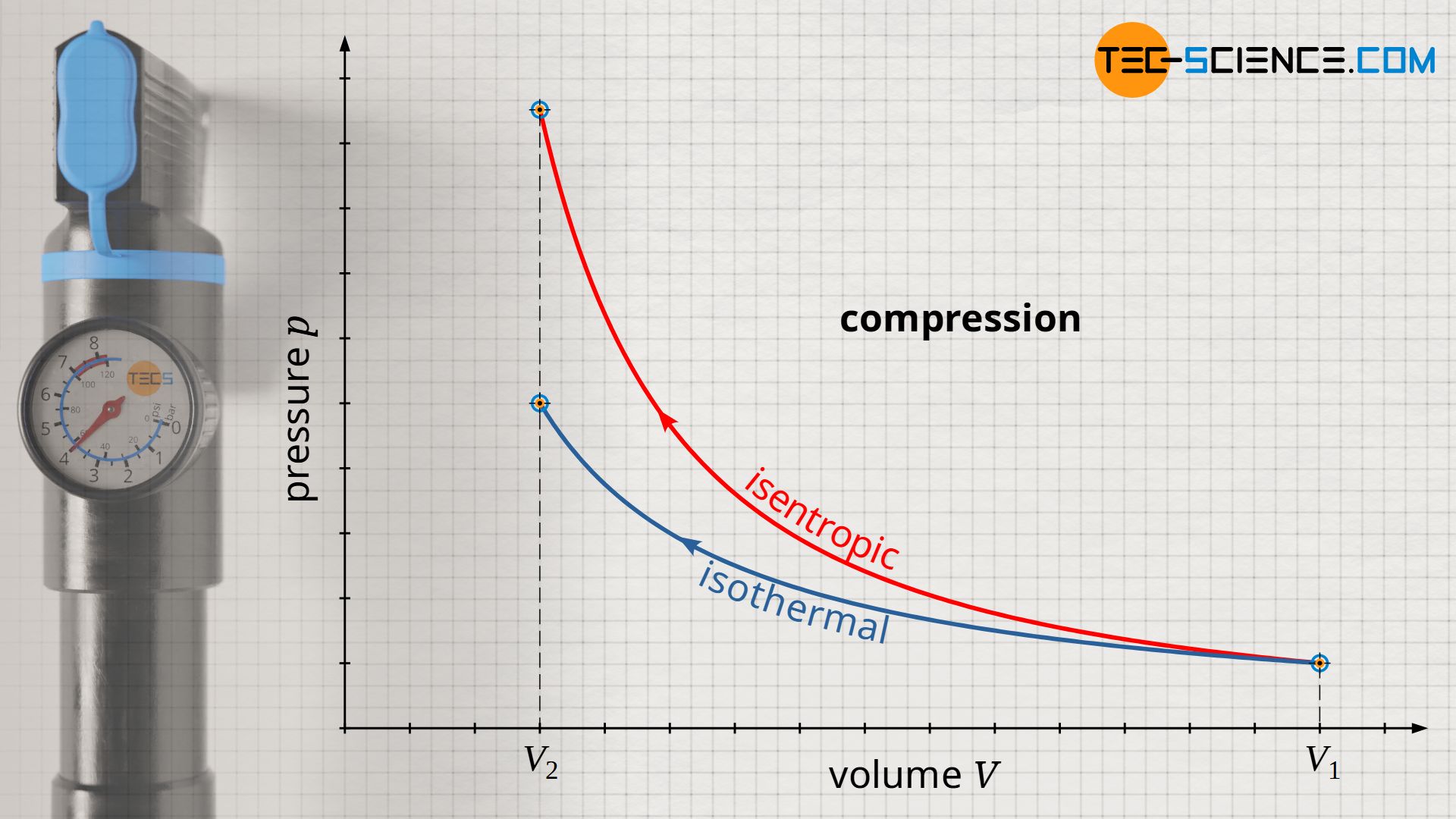
Isentropic (adiabatic) process in a closed system - tec-science
tec-science, Autor bei tec-science
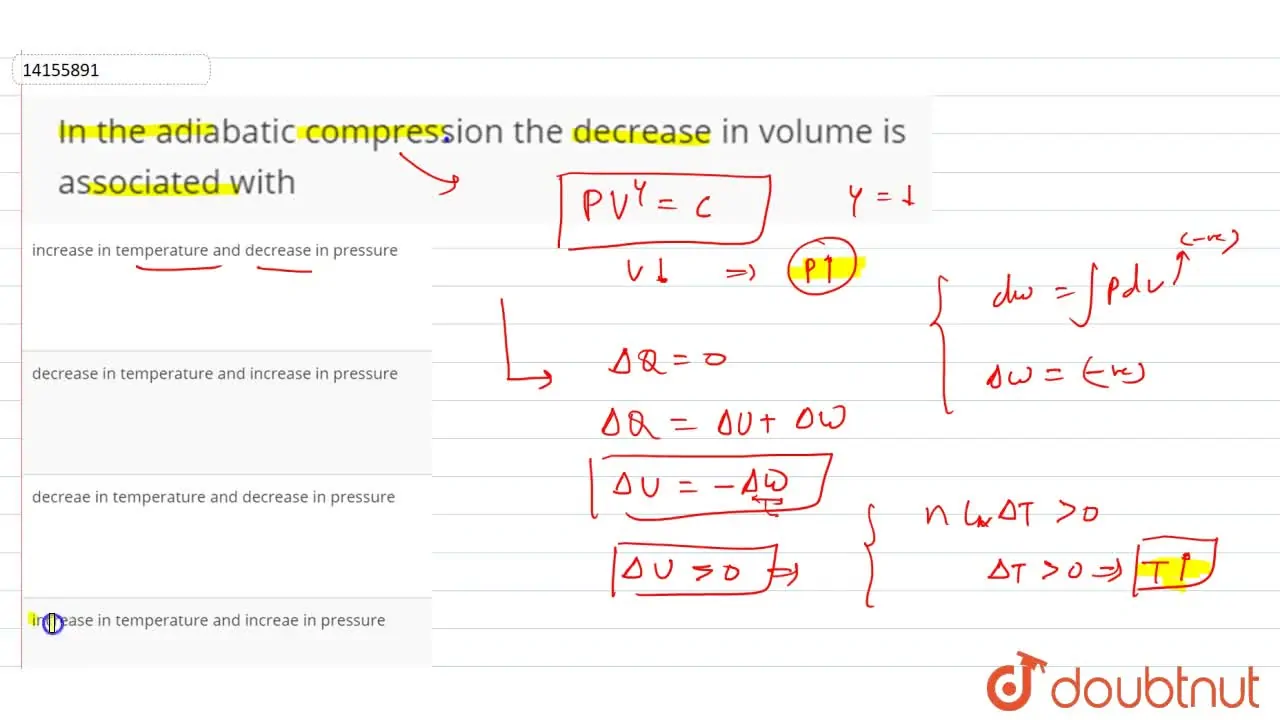
decreae in temperature and decrease in pressure

Dew Point vs Relative Humidity in Compressed Air Systems – Fluid
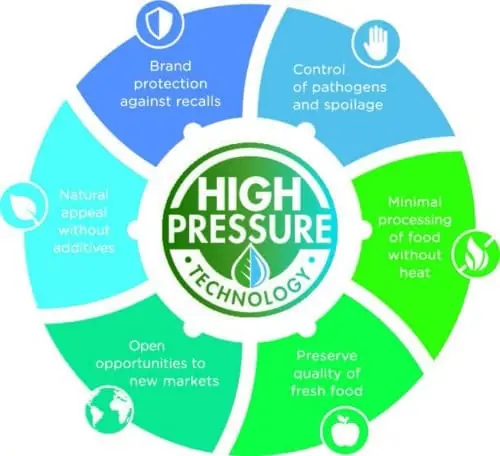
High Pressure Processing (HPP) Advantages - Hiperbaric

Lesson 9 COMPRESSION PROCESSES Apply the ideal gas laws to SOLVE

Why does water boil faster at high altitudes? - tec-science

Why does water boil faster at high altitudes? - tec-science

Thermodynamic processes

Air at 100 KPa and 280 K is compressed steadily to 600 KPa and 400

Why does water boil faster at high altitudes? - tec-science
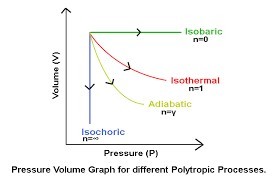
Adiabatic process PV^y = Constant, y = Cp/Cv = 3/2: A short