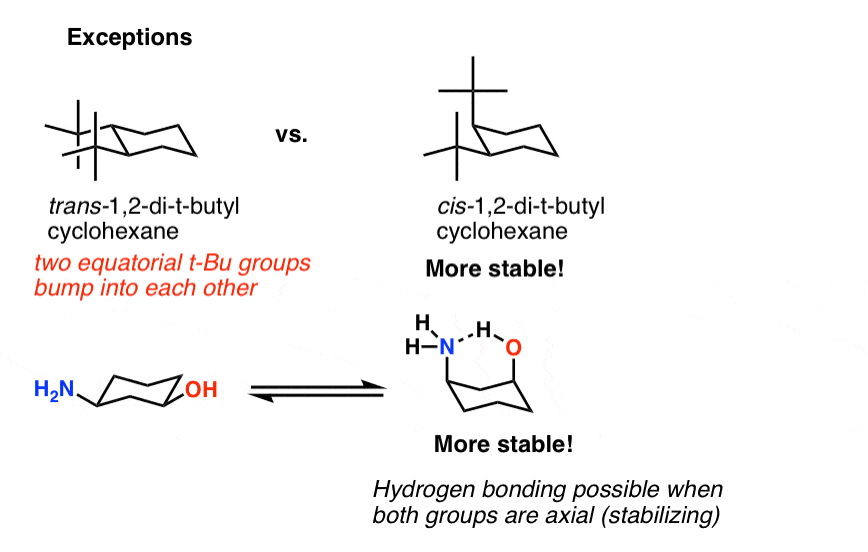
Cyclohexane Chair Conformation Stability: Which One Is Lower Energy?
By A Mystery Man Writer
To determine chair conformation stability, add up the "A-Values" for each axial substituent. The lower that number is, the more stable the chair.

Ch 3 : Cyclohexane
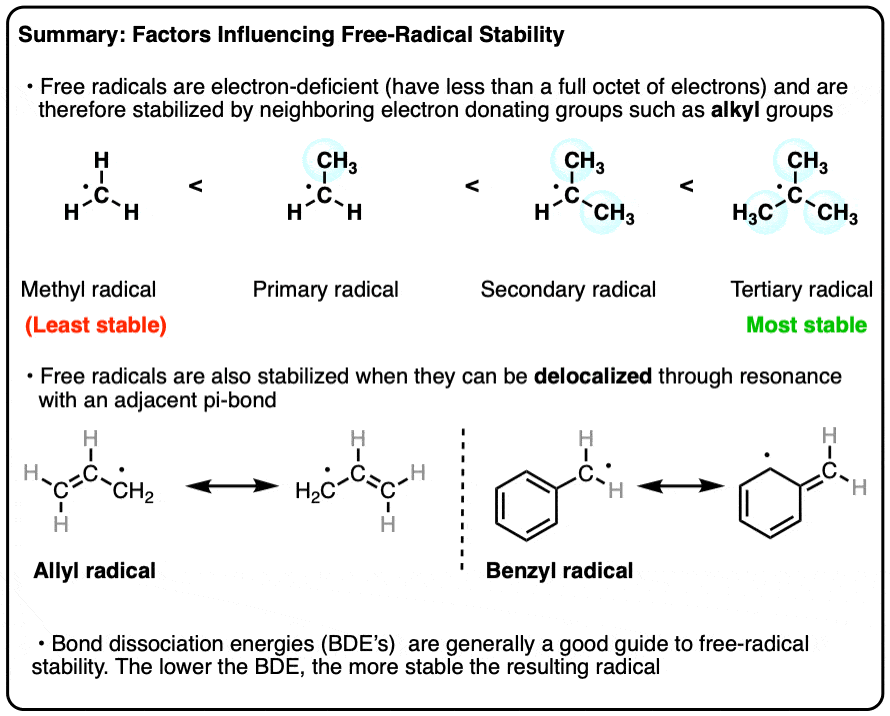
3 Factors That Stabilize Free Radicals – Master Organic Chemistry
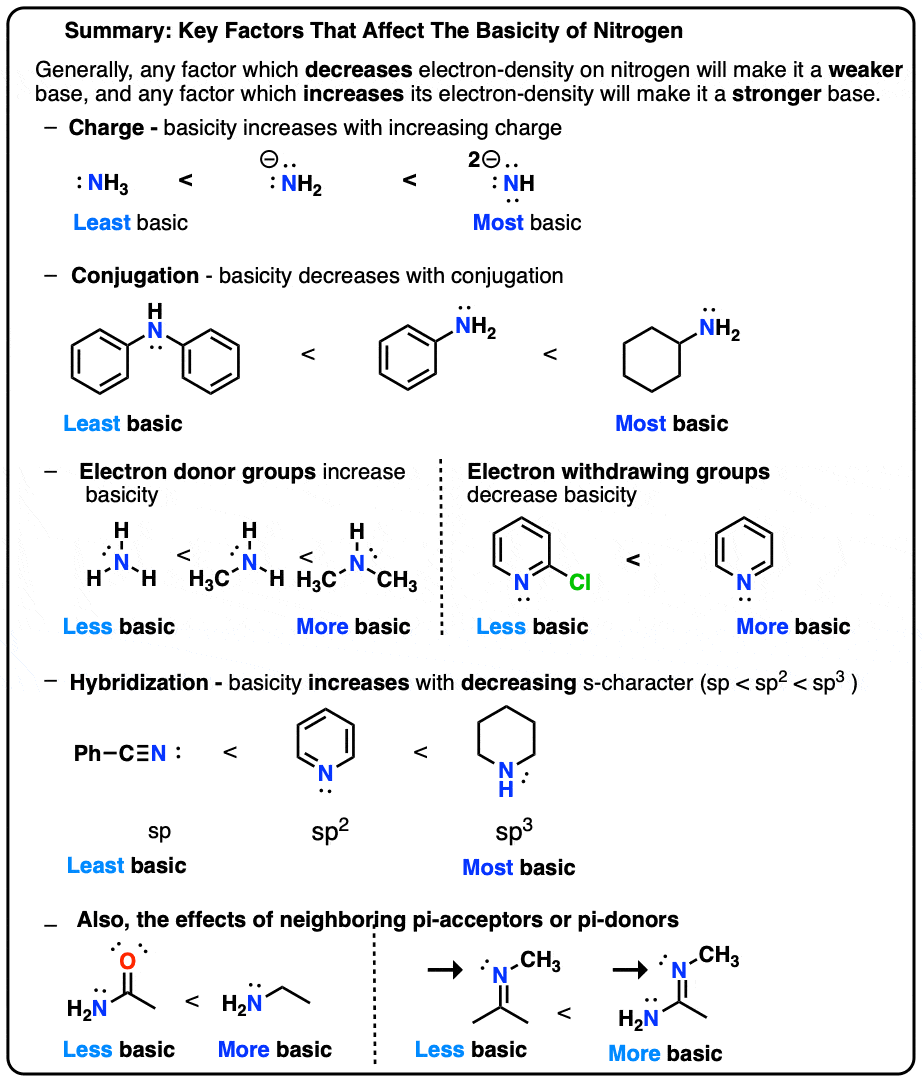
5 Key Basicity Trends of Amines – Master Organic Chemistry
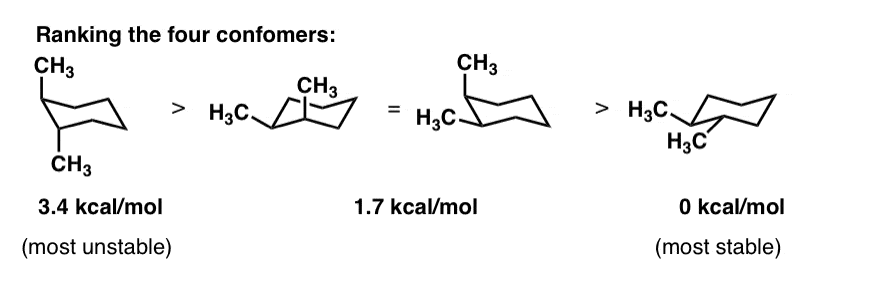
Cyclohexane Chair Conformation Stability: Which One Is Lower Energy?

The diaxial conformation of cis-1,3-dimethylcyclohexane is approximately 23 rac{kJ}{mol} (5.4 rac{kcal}{mol}) less stable than the diequatorial conformation. Draw the two possible chair conformations, and suggest a reason for the large energy

Draw the least stable Newman projection for isopropylcyclohexane.
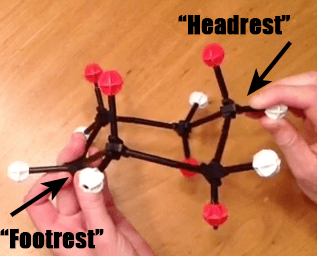
The Cyclohexane Chair Flip – Master Organic Chemistry

Cyclohexane conformation - Wikipedia
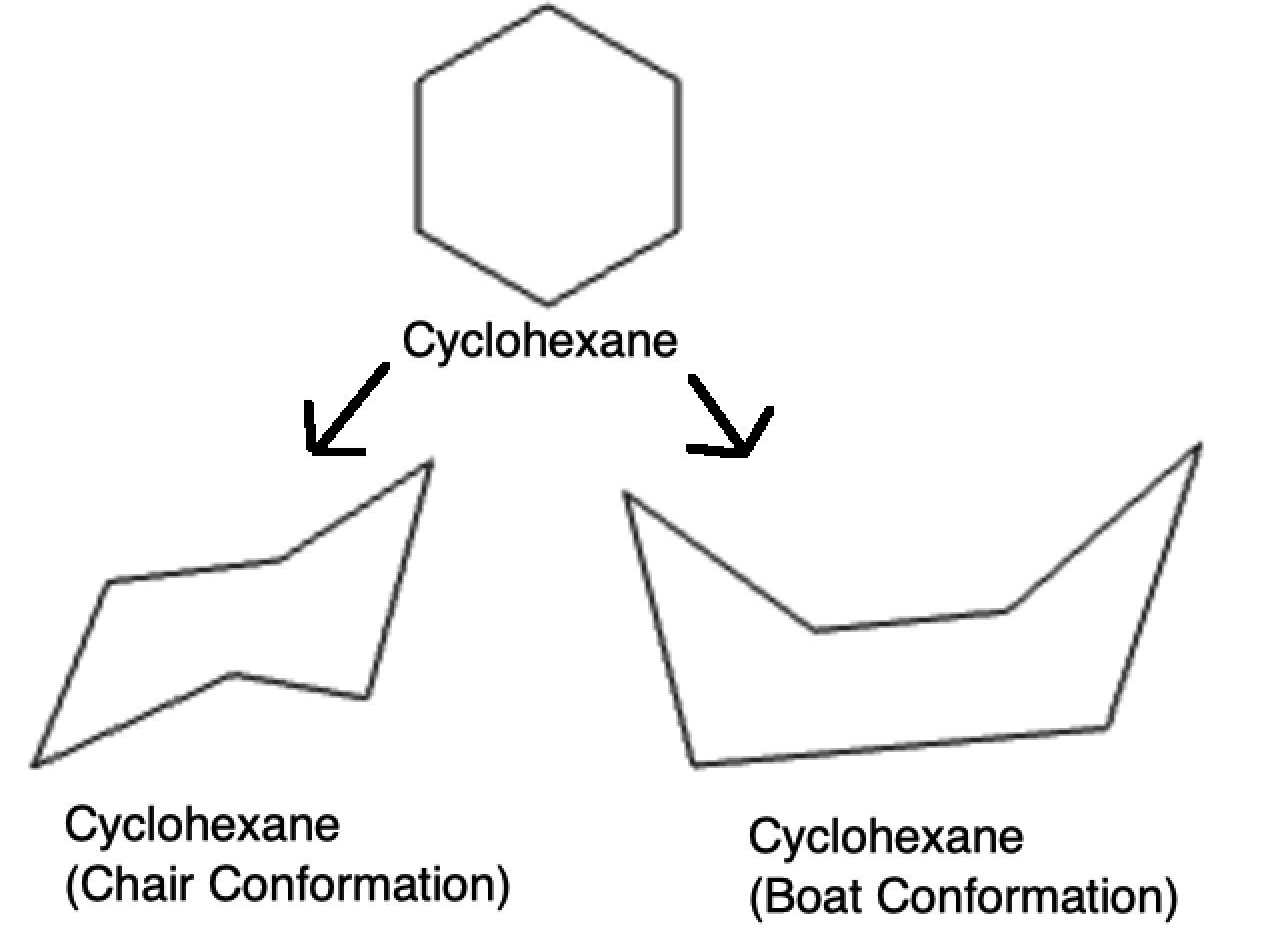
OrgoSolver
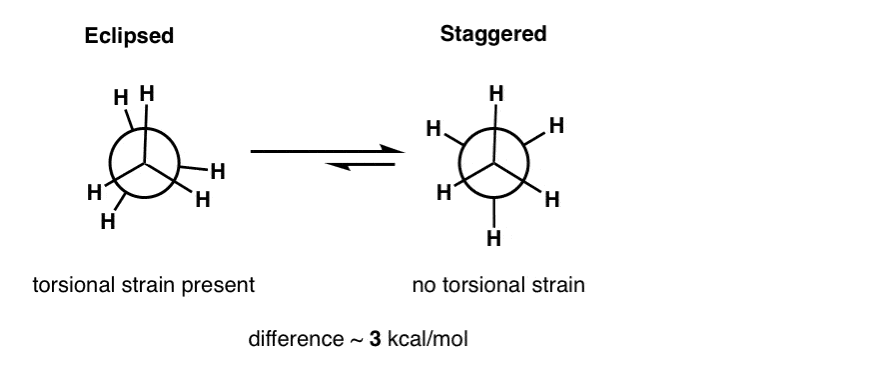
Cycloalkanes - Ring Strain In Cyclopropane And Cyclobutane
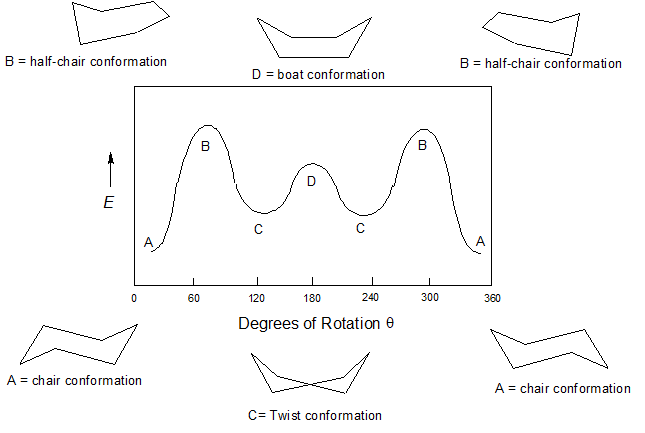
4.5: Conformations of Cyclohexane - Chemistry LibreTexts

3.6. Conformations of cyclic alkanes Organic Chemistry 1: An open textbook
- recent lululemon favorites - Lauren Kay Sims Lauren kay sims, Lululemon, Lululemon outfits

- Evening Wear & Matric Dance Dresses

- Shein Shein Curve Blouse Womens 3XL Red White Polka Dot Delicate Lightweight Soft
- Bebe Seamless Bra

- YIANNA Entrenador de cintura para mujer Corsé reductor de cintura debajo del busto de látex Faja deportiva Faja moldeadora de cuerpo con reloj de arena - Color Beige (25 huesos de acero)






