What is compressibility factor? What is its value for ideal gas

By A Mystery Man Writer

If a gas gets half compressed, compared to an ideal gas, the compressibility factor Z is equal to

Engg ThermodynamicsTwo Mark With Answer, PDF, Heat

1.7: Connecting the van der Waals and the viral equations: the Boyle temperature - Chemistry LibreTexts

Engg ThermodynamicsTwo Mark With Answer, PDF, Heat

Compressibility factor, Z of a gas is given as Z= frac { pV }{ nRT } (i) What is the value of Z an ideal gas?(ii) For real gas what will be

The compressibility factor of a gas is defined as Z=PV/nRT. The compressibility factor of an ideal gas is:1-1zeroinfinite
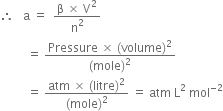
What is compressibility factor? What is its value for ideal gas

Engg ThermodynamicsTwo Mark With Answer, PDF, Heat

6.3: Van der Waals and Other Gases - Physics LibreTexts

Compressibility factor - Wikipedia
What is the value of compressibility factor for a non-ideal gas? - Quora
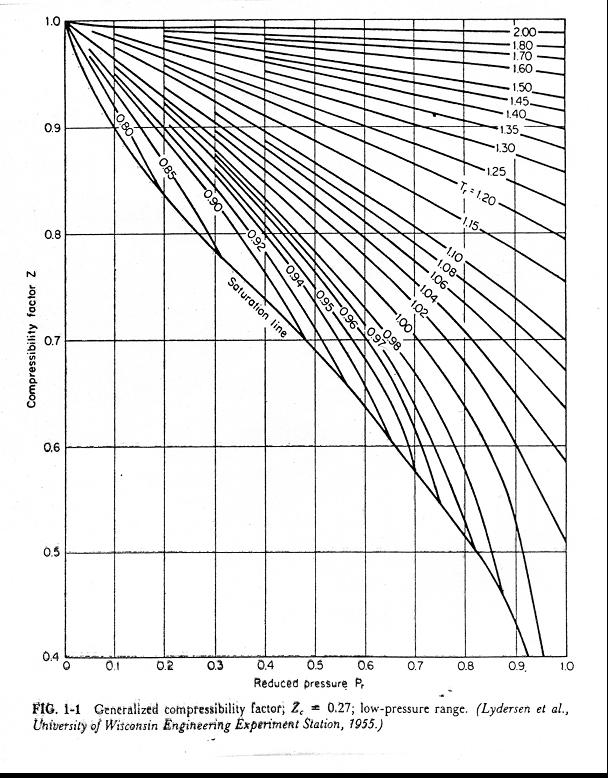
- Explain how the compression factor varies with pressure and

- 3.2 Real gas and compressibility factor – Introduction to

- The role of the compressibility factor Z in describing the
- physical chemistry - Why do some gases have lower value of Z for a

- Write the expression for the compressibility factor (Z) for one

- Skims lounge pants for Women

- Lucky Brand, Shirts, Lucky Brand 0 Linen Mens Large Blue Button Down Shirt

- PENGXIANG Waist Bag Ergonomic Hidden Pockets Moisture-Wicking

- Pearl Izumi Summit AmFIB Lite Pants (Phantom) (30) - Performance

- Whaline 24 Sheet St. Patrick Green Plaid Pattern Paper Pack Scrapbook Specialty Paper Double-Sided Collection Decorative Craft Paper for St. Patrick's
