The entropy change for the conversion of 36 g water to vapour at
By A Mystery Man Writer
The entropy change for the conversion of 36 g water to vapour at its boiling point at 1 atm is (Enthalpy of vaporization for water is 40.63 kJ mol–1)
The entropy change for the conversion of 36 g water to vapour at its boiling point at 1 atm is -Enthalpy of vaporization for water is 40-63 kJ mol-1
The free energy and entropy change in kJ per mole whenliquid water boils at 1 atmosphere are respectively(latent heat of water= 2.0723 kJ g^ 1) (a) 0, 0 (b) 0.1, 0.1 (c) 0.1,0 (d) 0, 0.1
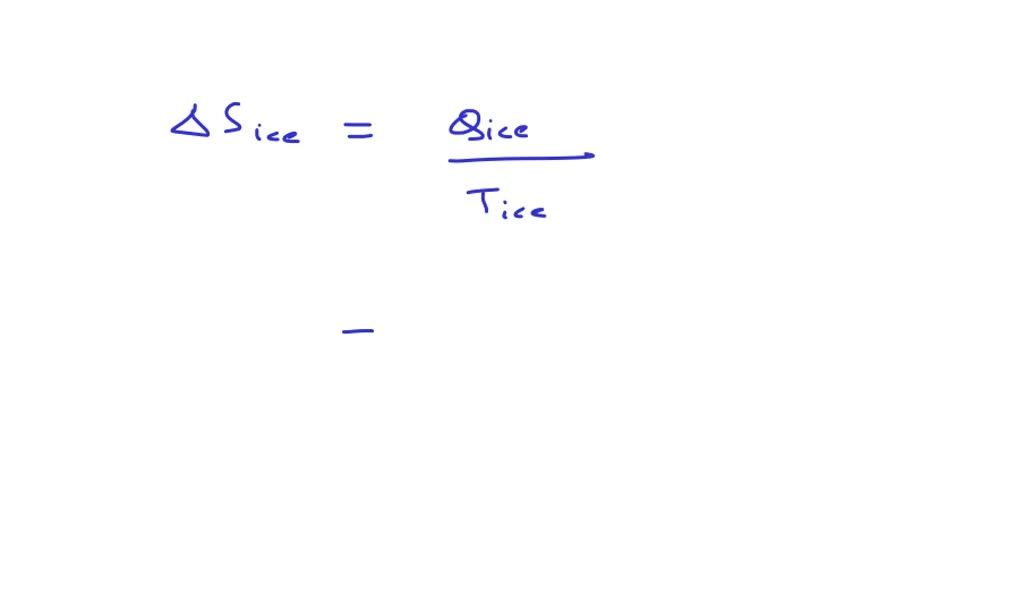
SOLVED: A block of ice at 273K is put in thermal contact with a container of steam at 373K, converting 25.0g of ice to water at 273K while condensing some of the
What is the change in entropy of 320g of steam at 100°C when it is condensed to water at 100°C? - Quora

The entropy change associated with the conversion of 1 kg of ice 273 K to water vapours 383 Kis: (specific heat of water liquid and water vapour are 4.2 kJ K-kg- and

The entropy change associated with the conversion of 1 kg of ice at 273 K to water vapours at 38
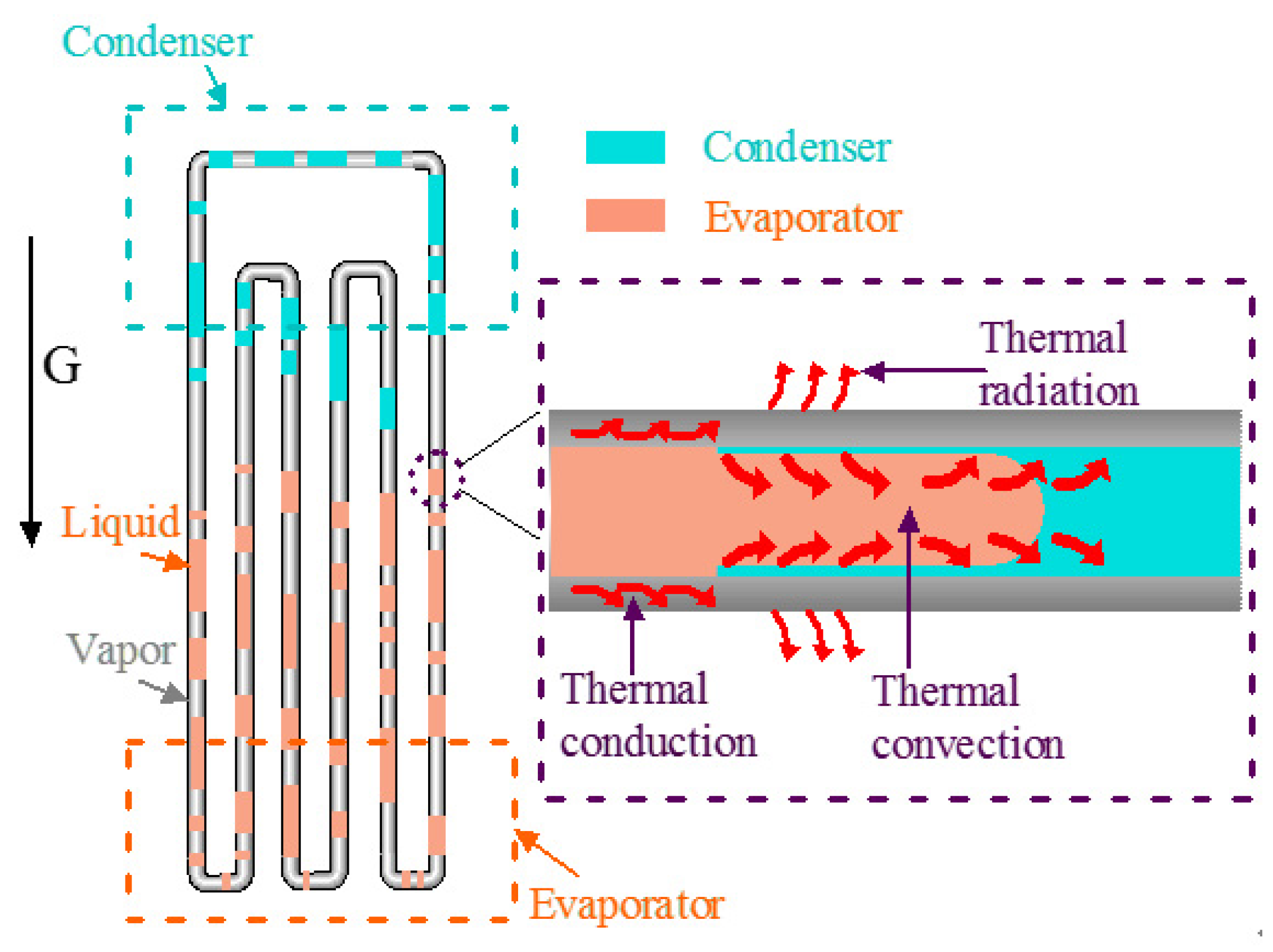
Energies, Free Full-Text

calculate the entropy change involved in conversion of one mole (18g) of solid ice at 273 K of liquid water - Chemistry - Thermodynamics - 9709217
What is the entropy change in going from vapour to liquid state at any temperature? - Quora

Calculate the entropy change of n-hexane when 1 mole of it evaporates
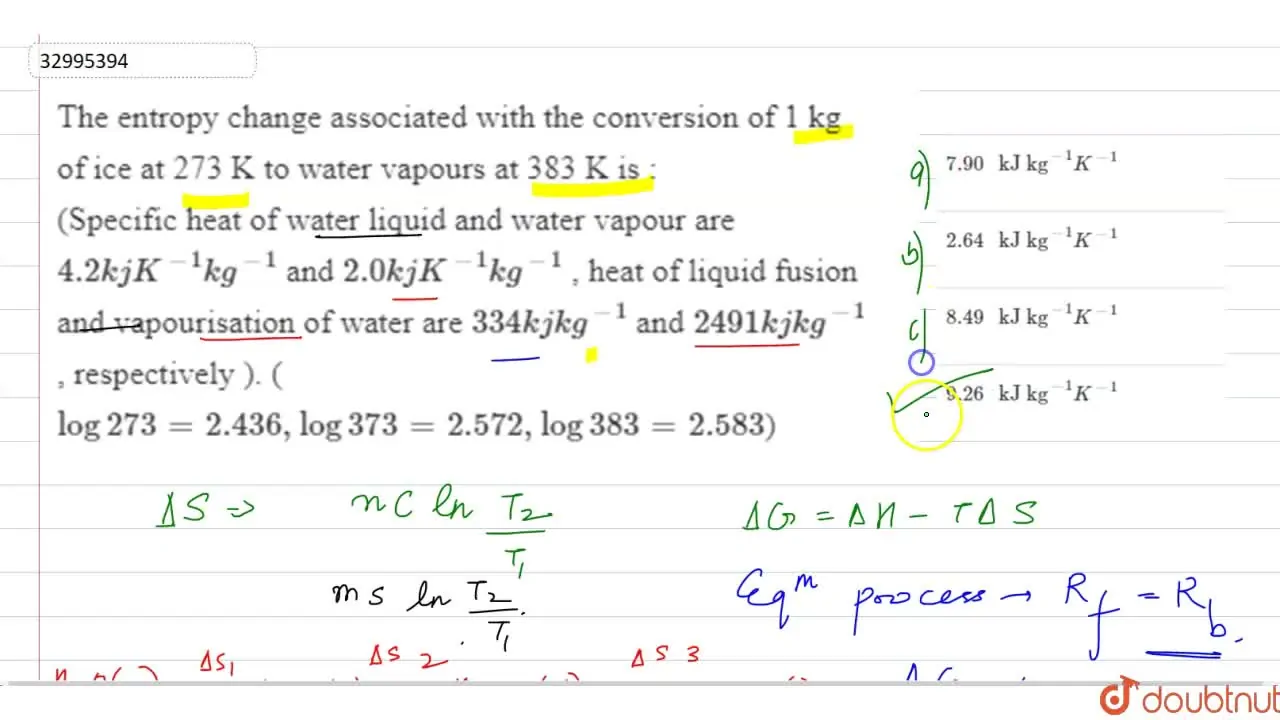
The entropy change associated with the conversion of 1 kg of ice at 27

3) 6025 JAK (4) 602.5 JIK 87. Calculate the entropy change the conversion of 36 g water to vapour 373 K; AH, HO=40.63 k mor! (2) 202.07 JAK () 602 JK (4)

66. The entropy change for the conversion of 36 g of water to vapour at 100°C (Normal boiling point) is
- Divisora De Massas DV-36 - G.Paniz - Monte Alegre Refrigeração

- Lindt Lindor Milk Chocolate Truffles Box, 12 × 36 g
- Whey Pro 36g de Proteina Body Nutry Pacote 900g - Ganho de Massa

- Fantasie Aubree Uw Side Support Bra 36 G - Rinnahoidjad

- Biscoito Bauducco ChocoBiscuit ao leite 36g - Gmaxx Distribuidora: Tudo para o seu comércio em um só lugar.

- I had so much fun with the @Knotty Official team today! They're all so
- 2-pack Thick Jersey Leggings - Navy blue/hearts - Kids

- 1 6 Scale Action Figure Head Sculpt Male Pvc Male Head Carving Model - 1/6 Scale - Aliexpress

- Terra & Sky Women's Plus Size … curated on LTK
- CHANTELLE Soft Stretch High Waist Mid-thigh Short - Shapewear


