Solved RT B 2. The compressiblity factor for a gas is

By A Mystery Man Writer
Answer to Solved RT B 2. The compressiblity factor for a gas is

1.7: Connecting the van der Waals and the viral equations: the Boyle temperature - Chemistry LibreTexts

Solved] The compressibility factor for an ideal gas is
JEE - Compressibility Factor Important Concepts and Tips

Cubic equations of state - Wikipedia

The equation of state of a real gas is p(v-b) =RT Can the gas be liquefied? Explain? - EduRev Class 11 Question

Determine Compressibility of Gases

A certain gas obeys P(Vₘ - b) = RT. The value of (∂Z/∂P)ₜ is xb

Compressibility Factor of Gas, Overview, Equation & Chart - Lesson
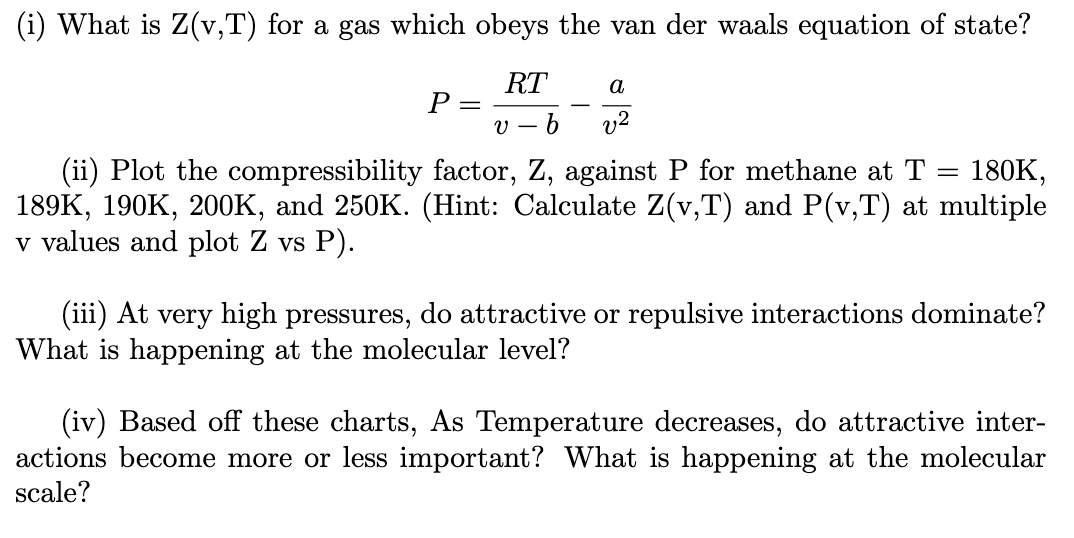
Solved (i) What is Z(v,T) for a gas which obeys the van der

Deviation of Real Gases from Ideal Gas Behaviour - GeeksforGeeks

Compressibility Chart - an overview
- Thermodynamics - 3-7 Ideal Gas Equation with compressibility factor example 1

- Ideal Gas Equation and COMPRESSIBILITY Factor in 11 Minutes!

- Slope of graph of compressibility factor(Z) with pressure(P) for hydrogen gas at any pressure i
- 3.3.3: Natural Gas Properties PNG 301: Introduction to Petroleum and Natural Gas Engineering
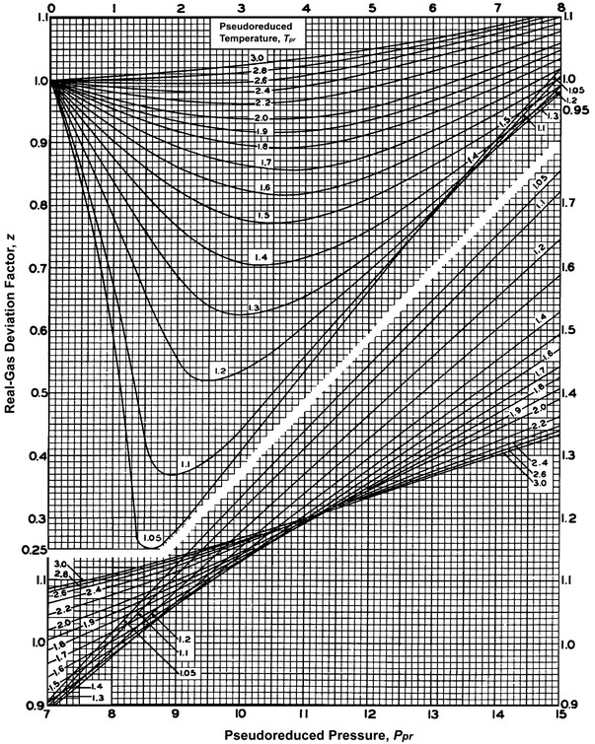
- At Critical Temperature,pressure and volume . The compressibility
- Koala Makeup Bag Gifts Koala Girls Gifts Women Koala Things - Temu

- Pogamat Large Yoga Mats - 78 X 27 X 6.5mm Extra Thick Fitness Mat – High Density Anti-Tear Exercise Mat For Yoga And P90X. Best Guys and Girls Yoga

- John Lewis and Waitrose announce price reduction for period underwear

- How to choose Ethnic Wear for Teen Girls? Tips and Guidelines

- Freya Womens Active Underwire Moulded Sports Bra
