Subfascial‐located contraceptive devices requiring surgical removal, Contraception and Reproductive Medicine
By A Mystery Man Writer
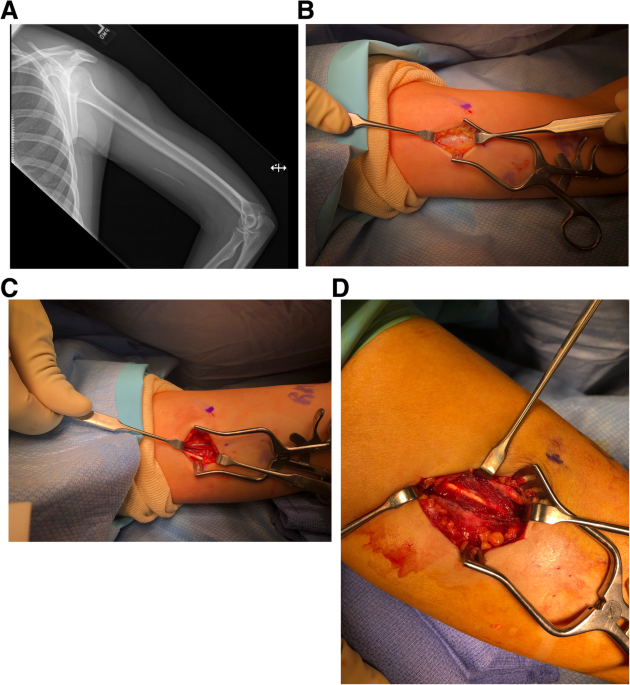
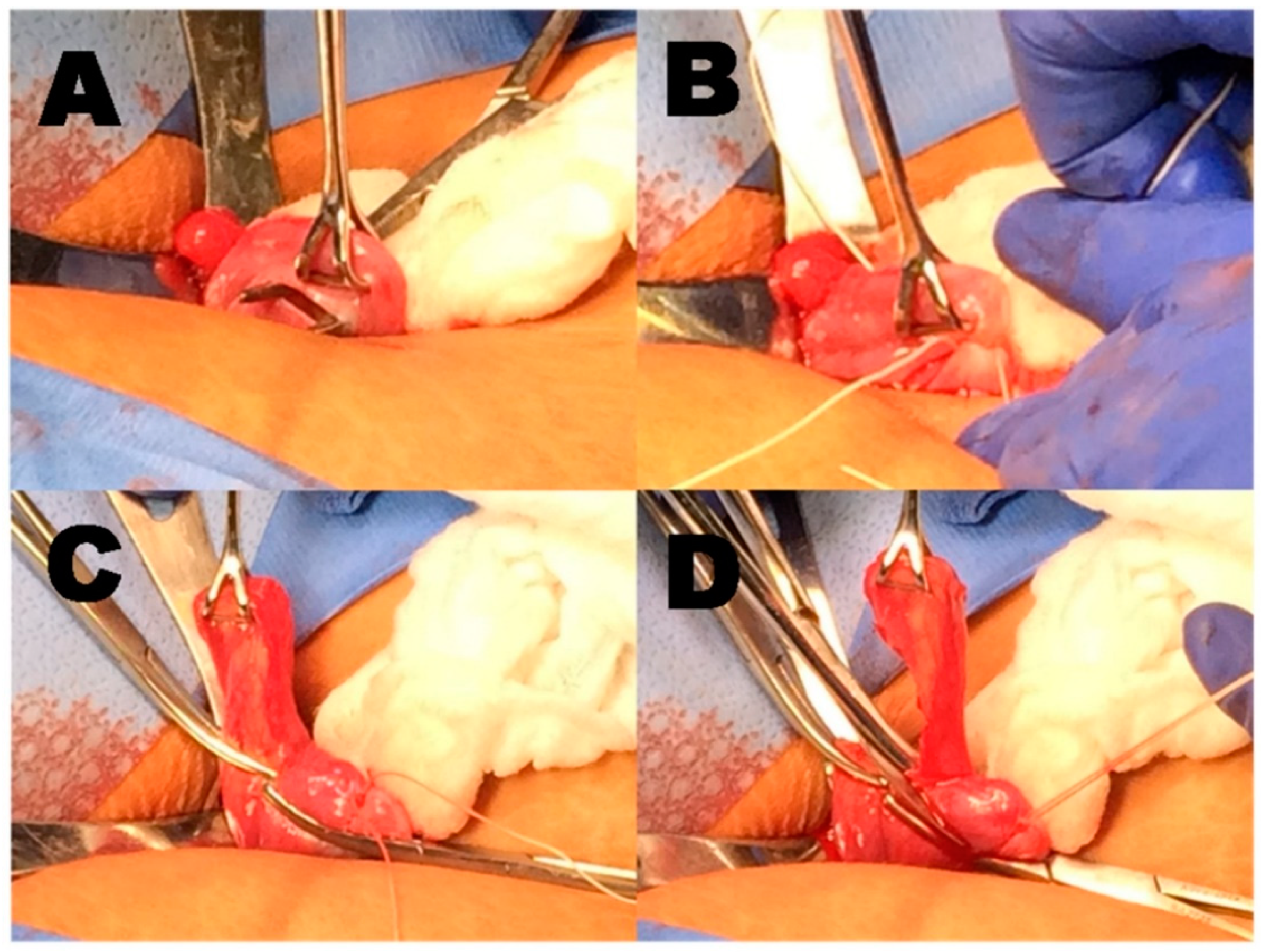
Background Subdermal etonogestrel implants are highly effective contraceptive methods. Despite standardization of insertion technique by the manufacturer, some implants are inadvertently placed too deeply within or below the plane of the biceps brachii fascia. Placement of these implants in a deep tissue plane results in more difficult removal, which is not always possible in the office setting. In rare cases, surgical removal by an upper extremity surgeon is warranted. Case presentation Here we present 6 cases of etonogestrel implants located in a subfascial plane requiring removal by an upper extremity surgeon. Implants were all localized with plain radiography and ultrasound prior to surgical removal. All cases had implants located in the subfascial plane and one was identified intramuscularly. The average age was 28 years (19–33) and BMI was 24.0 kg/m^2 (19.1–36.5), with the most common reason for removal being irregular bleeding. The majority of cases (5/6) were performed under monitored anesthesia care with local anesthetic and one case utilized regional anesthesia. All implants were surgically removed without complication. Conclusions Insertion of etonogestrel contraceptive implants deep to the biceps brachii fascia is a rare, but dangerous complication. Removal of these implants is not always successful in the office setting and referral to an upper extremity surgeon is necessary to avoid damage to delicate neurovascular structures for safe removal.

Difficult removal of subdermal contraceptive implants: a multidisciplinary approach involving a peripheral nerve expert.
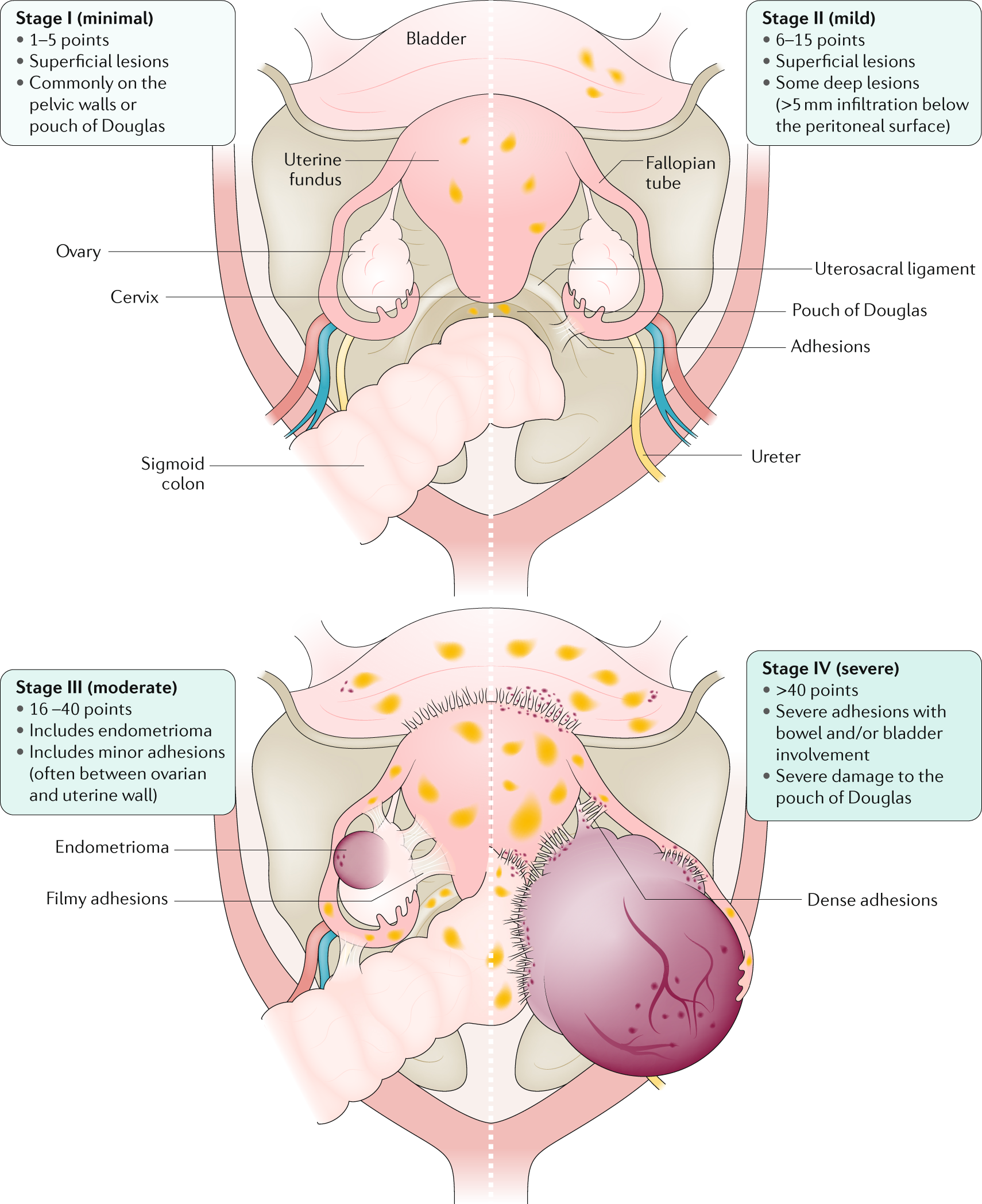
Endometriosis Nature Reviews Disease Primers

Contraception and Reproductive Planning for Women With Cardiovascular Disease: JACC Focus Seminar 5/5 - ScienceDirect
Nonhormonal Contraception
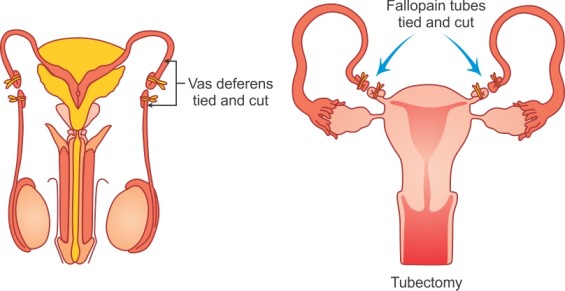
Family Planning - Nurses Revision

IUD perforation and embedment within omentum: a rare and perplexing incidence

PDF) Subfascial‐located contraceptive devices requiring surgical removal
Reprod. Med., Free Full-Text
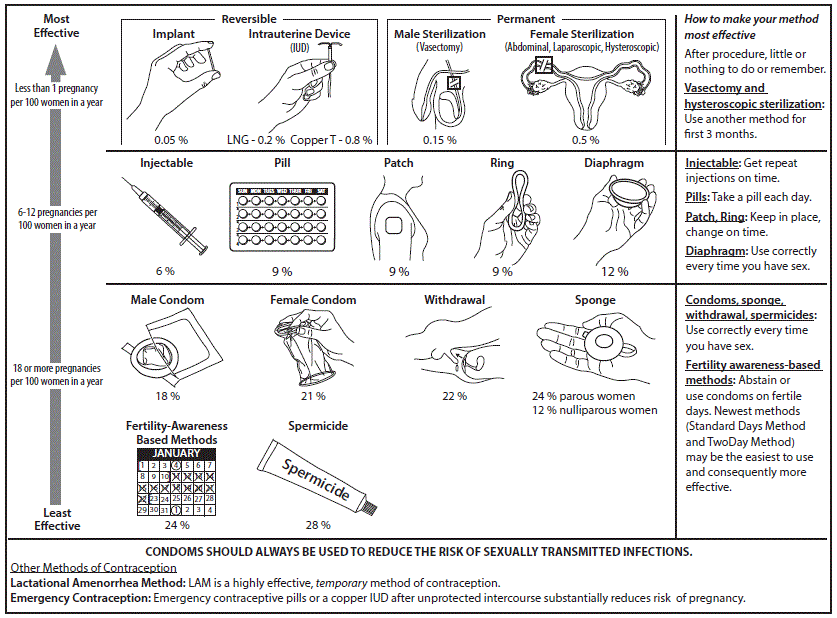
U.S. Medical Eligibility Criteria for Contraceptive Use, 2016
- Removal of an intramuscularly positioned implant near the brachial

- Breast Implant Removal Miami - Breast Explant Miami - Dr. Nirmal Nathan
- En Bloc Breast Implant Removal - Dr. Pancholi
- Reproductive Health Access Project Contraceptive Pearl: Implant Removal: Pop Out Technique - Reproductive Health Access Project
- Your Options For Silicone Implant Removal

- Action Figure One Piece - Nami Kung Fu Glitter & Glamours Blue

- tenis_esportivo] [nike_adidas_asics_under_aurmour] [tenis

- Lumento Women Comfy Sleepwear Pajama Shorts Casual Mid Waist Camo Lounge Shorts PJ Sleep Bottom Shorts S-XXL Dark Gray S(US 4-6)

- Don't Stop Me Now - Wikipedia

- Buy Zivame Innovation Push-Up Non Wired Medium Coverage Bra
