Solved) - NRT The Ideal Gas Equation States That Pi Where P Is The Pressure, (1 Answer)
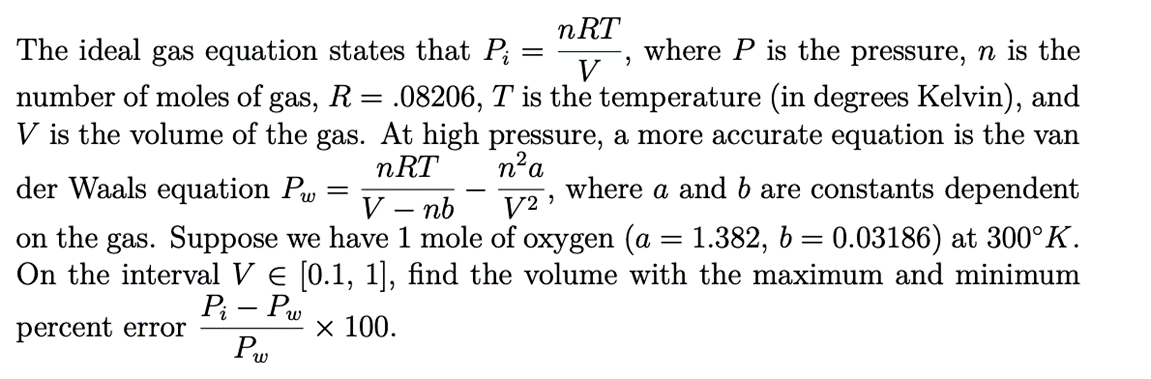
By A Mystery Man Writer
amp;#160;NRT The Ideal Gas Equation States That Pi Where P Is The Pressure, N Is The V Number Of Moles Of Gas, R= .08206, T Is The Temperature (In Degrees Kelvin), And V Is The Volume Of The Gas. At High Pressure, A More Accurate Equation Is The Van NRT
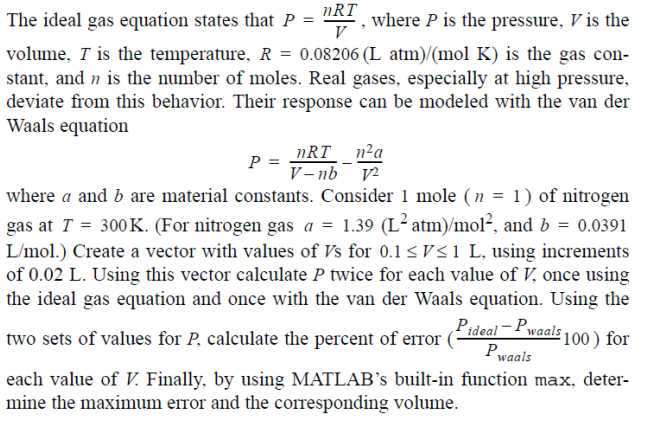
Solved nRT The ideal gas equation states that P = , where P
Processes, Free Full-Text
1st law
How to prove ideal gas law(pv=RT) - Quora
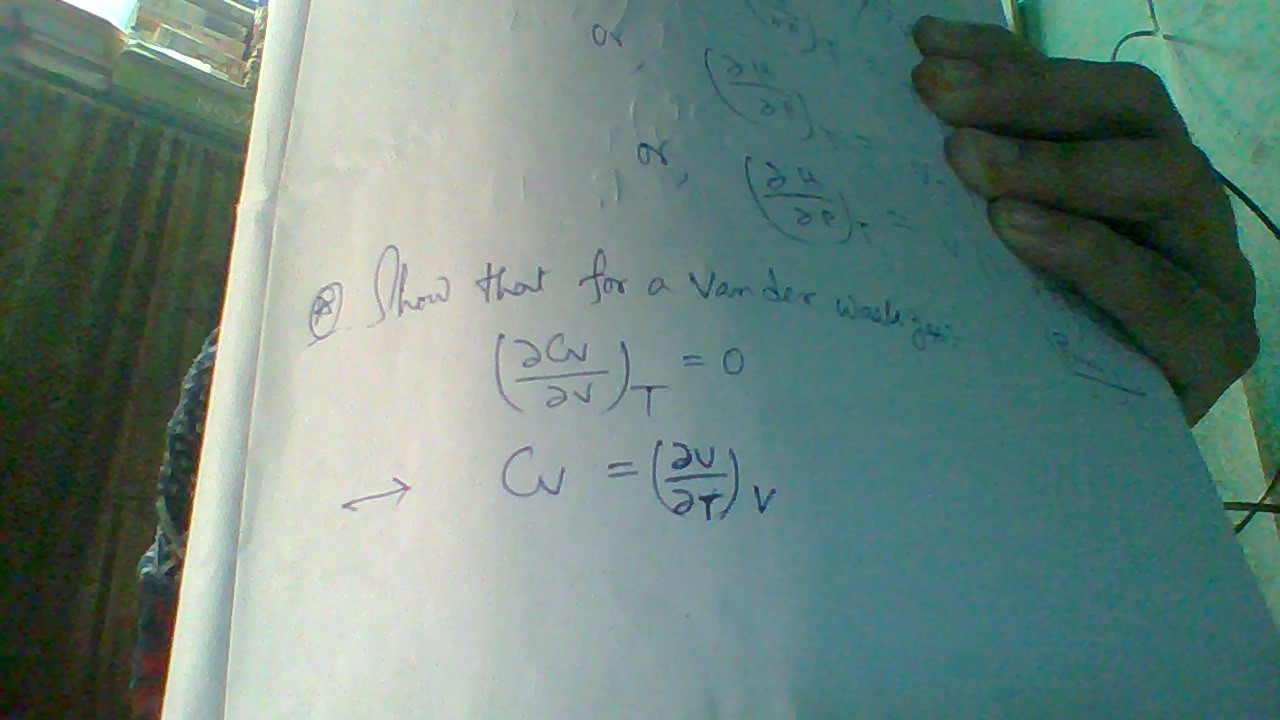
Show that for a van der Waals gas, ((delC_V)/(delV))_T = 0, where C_V = ((delU)/(delT))_V?

Gas Laws - Equations and Formulas
Equation of state - Wikipedia

Kinetic Theory Of Gases - Notes - LearnPick India
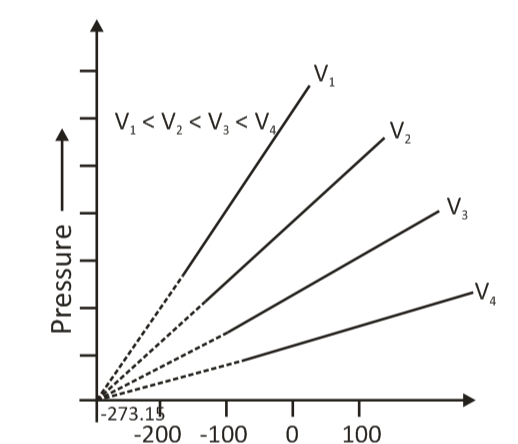
Gay Lussac's Law of Thermodynamics

thermodynamics - What happens to the $PV=nRT$ formula as the gas
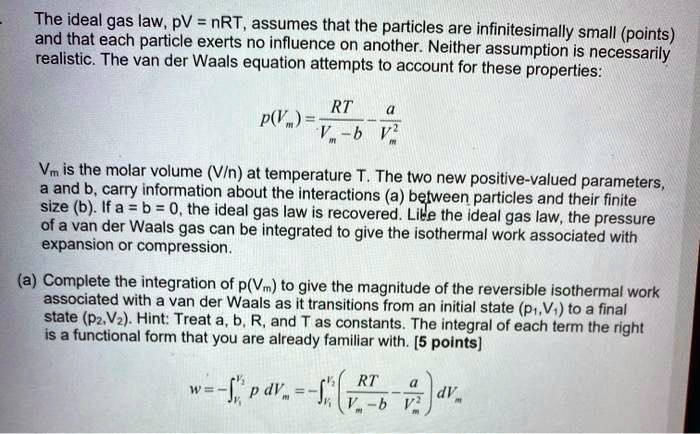
SOLVED: The ideal gas law, PV = nRT, assumes that the particles

Ideal Gas Law - an overview

PPT - Chapter 5: Gases and the Kinetic - Molecular Theory
What is the equation for calculating the pressure exerted by an

Ideal gas law - Wikipedia









