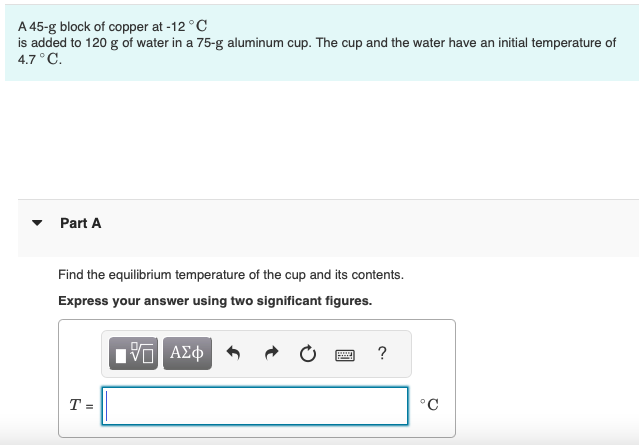
Solved A 45-g block of copper at −12∘C is added to 120 g of
By A Mystery Man Writer
Answer to Solved A 45-g block of copper at −12∘C is added to 120 g of
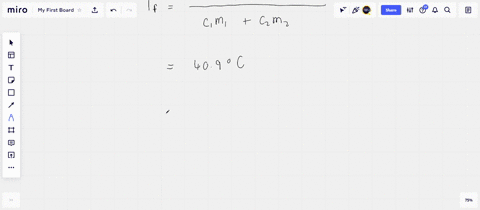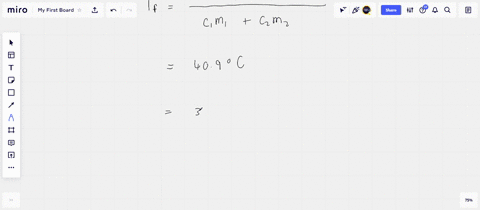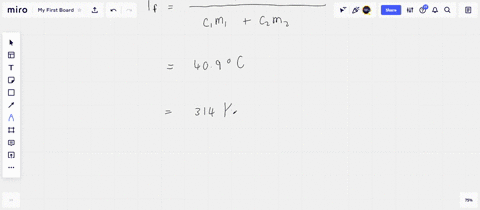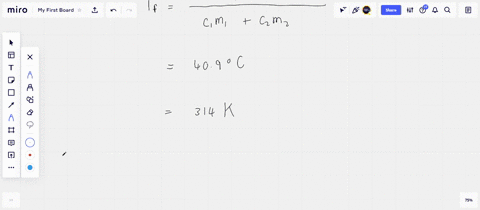
SOLVED:A 45.0 g block of tungsten at 30.0^∘ C and a 25.0 g block…
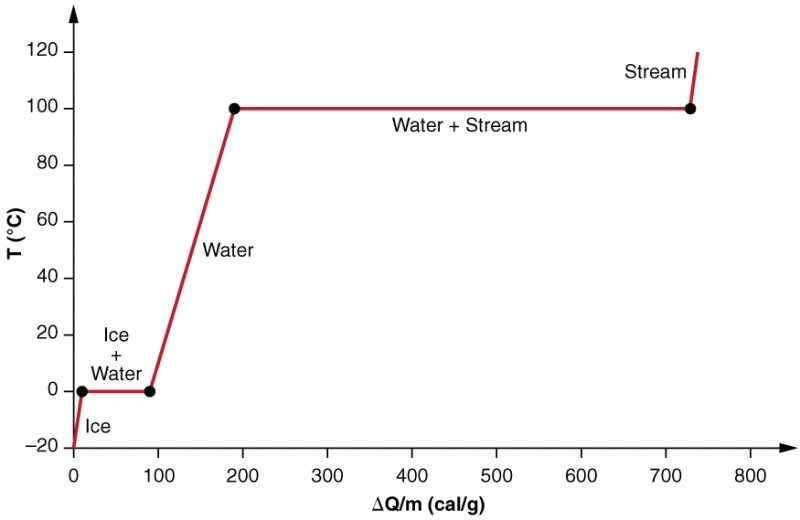
Phase Change and Latent Heat

5.22 A 70.0-g piece of metal at 80.0 °C is placed in 100 g of water at 22.0 °C contained in a

Temperature Change and Heat Capacity

Gold - Wikipedia
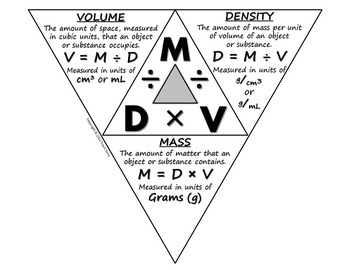
Density - Chemistry
45 g of water at 50∘C in a beaker is cooled when 50 g of copper at 18∘C is added to it.The contents are stirred till a final constant temperature is reached.Calculate
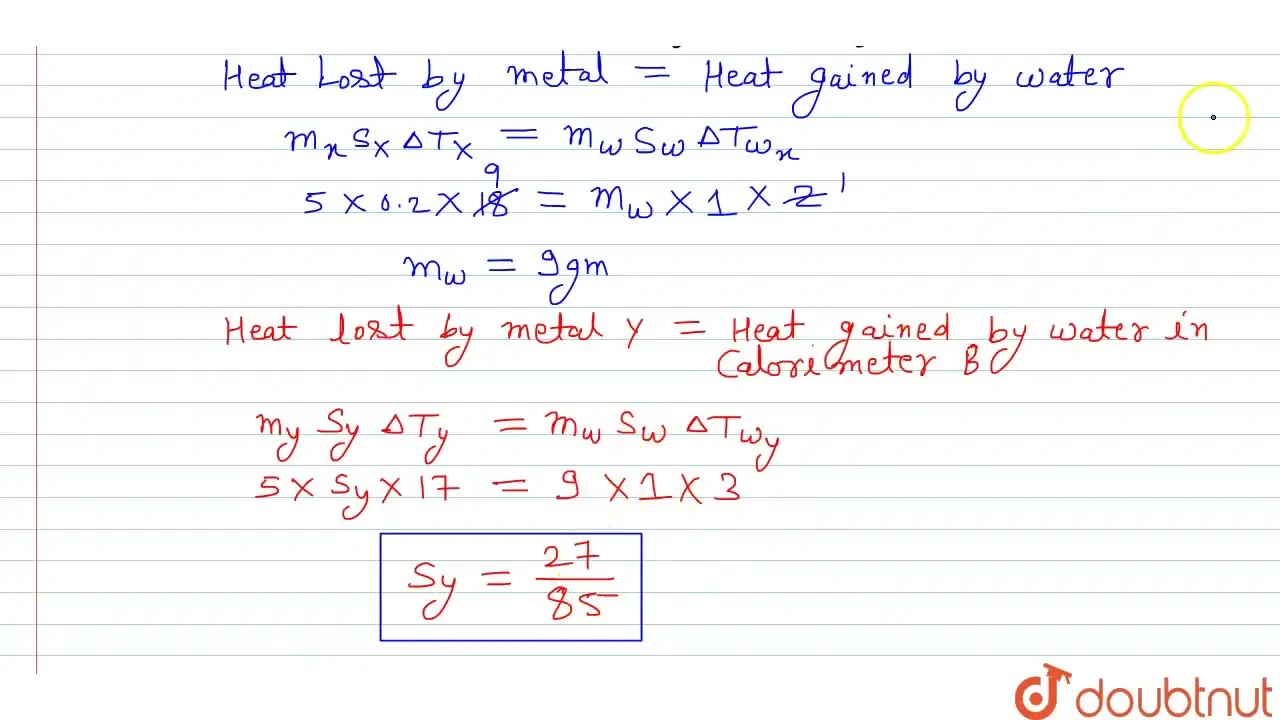
Two identical calorimeters A and B contain an equal quantity of water

Energies, Free Full-Text

Energies, Free Full-Text
2 kg of ice at 0°c is mixed with 8 kg of water at 20°c. What is the final temperature? - Quora

OpenStax College Physics, Chapter 14, Problem 13 (Problems & Exercises)
GI - Design and performance of the Hotrod melt-tip ice-drilling system
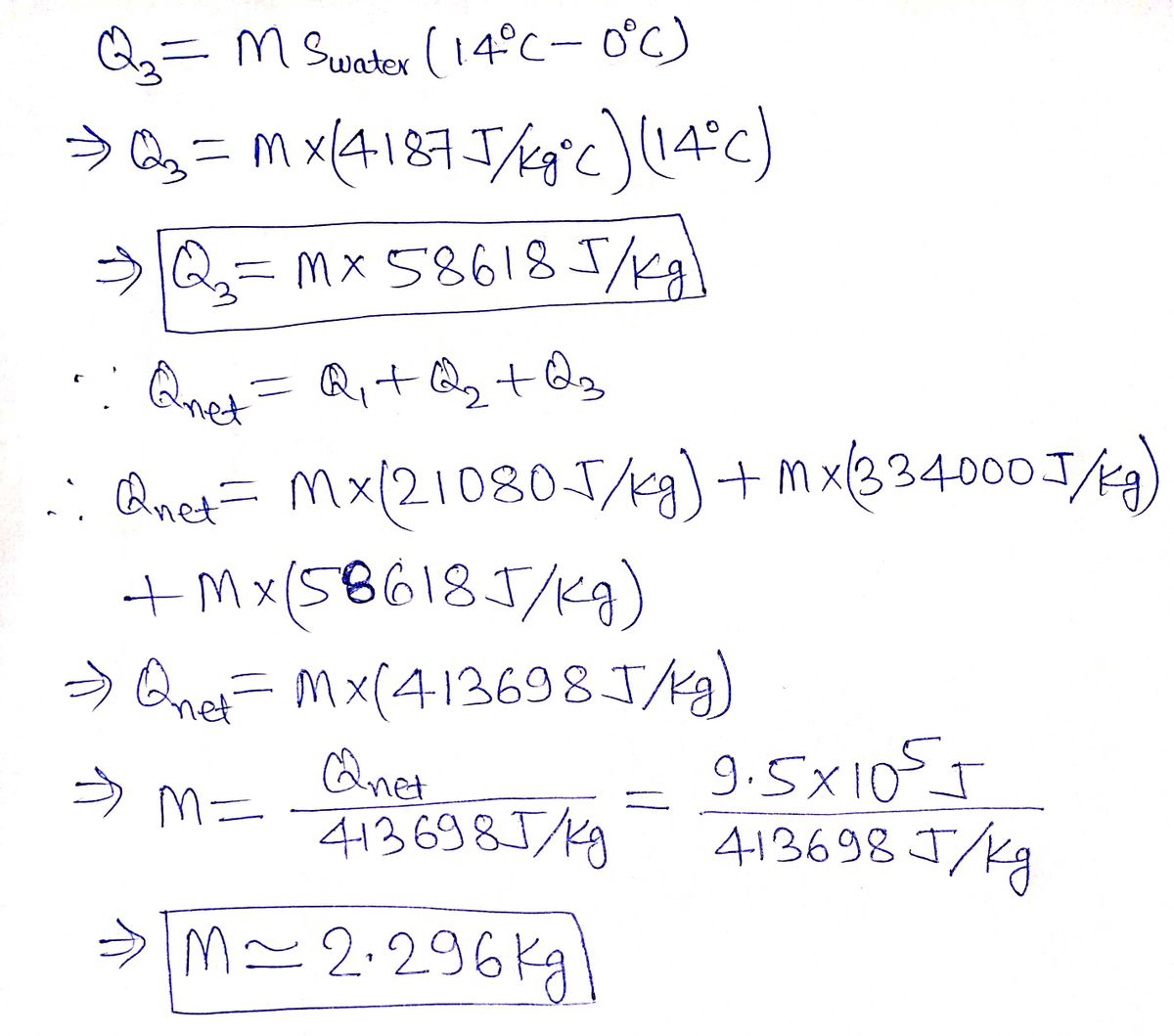
Answered: A heat transfer of 9.5×105 J is…
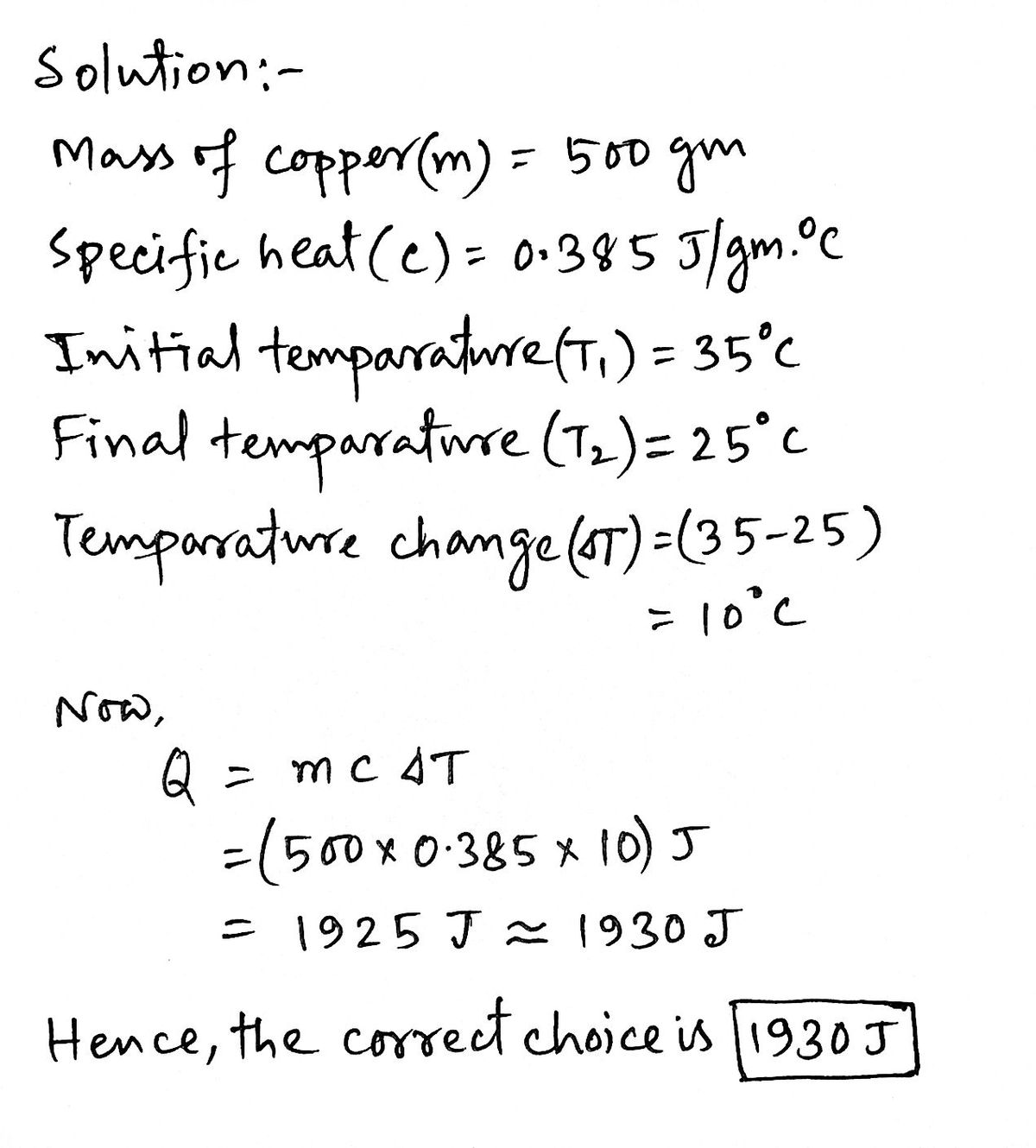
Answered: How much heat is absorbed when 500. g…
- Conversions made easy Cup conversion, Baking measurements, Baking conversions

- ACT Galvanneal - Hot Dip Zinc/Iron Alloy (45 g/m²) 0.94 x 39.5 x 0. - ACT Test Panels LLC

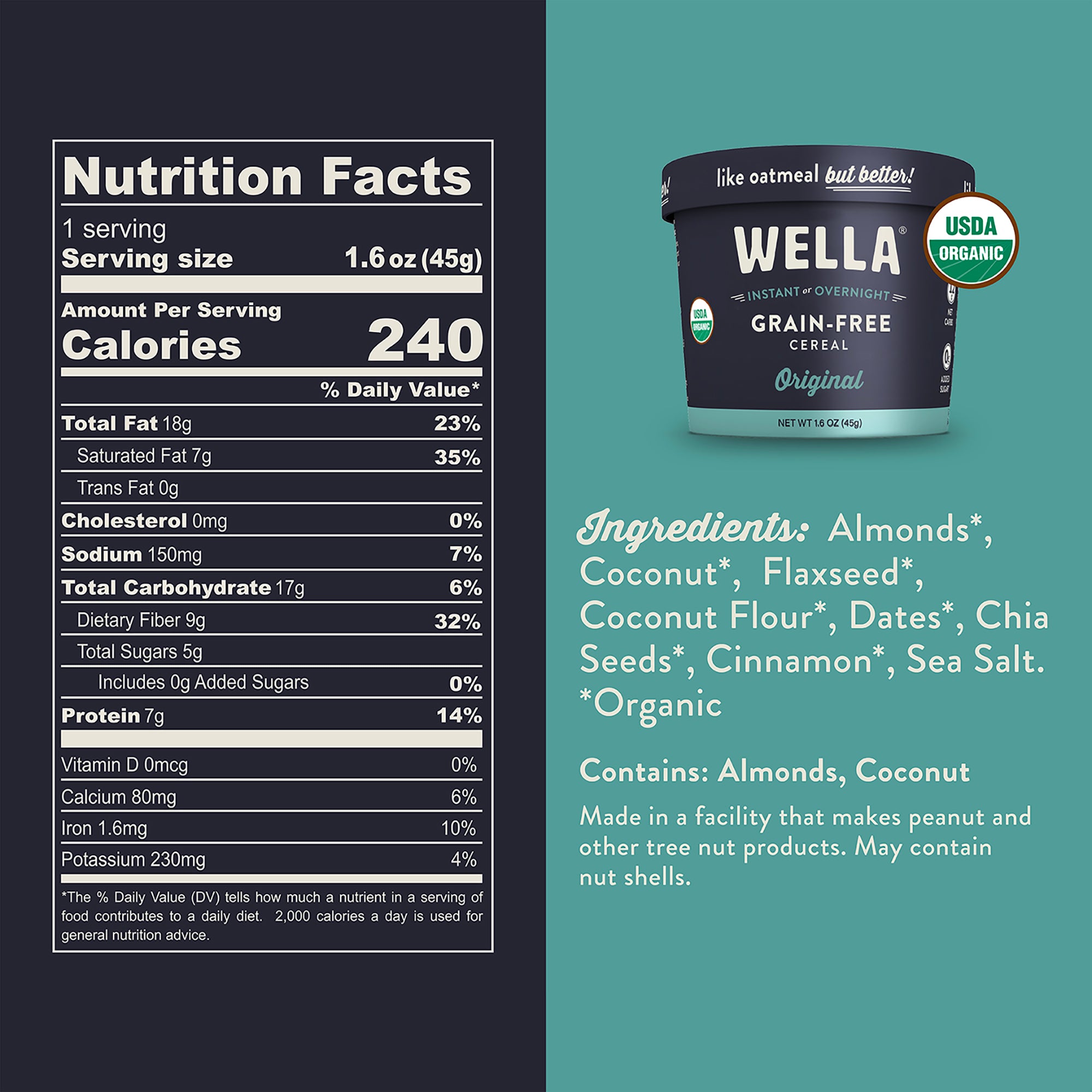
- Grain-Free Cereal Original Cups – 8 Count
- Drakes Online Findon - Continental Cream Of Chicken Simmer Soup
- Knorr Big Cup Instant Rice Porridge Chicken 45g. Pack 3

- Hot Nude Babes Naked Models Whippedwomen Whippedwomen Model

- HSMQHJWE Braless Tank Tops For Women Set Women'S Gradient Fit Slim Vest Low Collar Button Down Sleeveless Top Tank Fashion Slim Vest Blouses Comfy Tops Women

- Shop Swoosh Medium-Support Women's Padded Graphic Sports Bra

- Aayomet Lingerie for Women Plus Size Women's Traceless Lace Edge Bra Attractive Popular Big Chest Show Small Underwear Set,Black XL

- Sofa with triple evolution in moulded and carved walnut;…
