SOLVED: A 35-g ice cube at 0.0 °C is added to 110 g of water in a 62-g aluminum cup. The cup and the water have an initial temperature of 23 °C. (
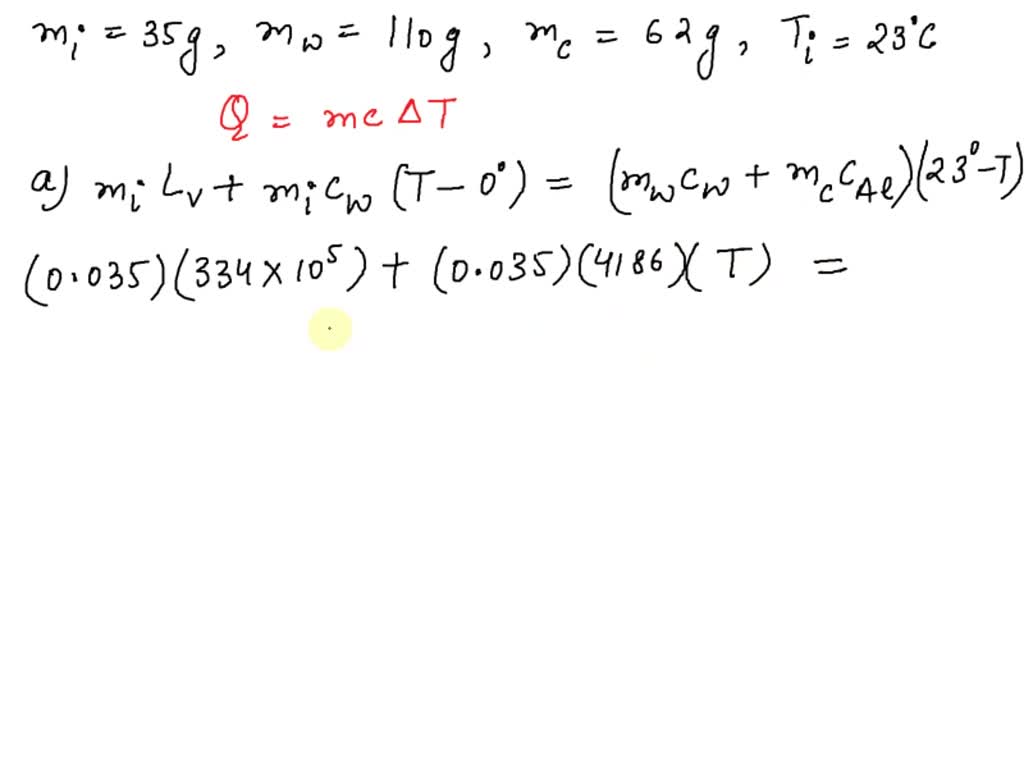
By A Mystery Man Writer
VIDEO ANSWER: Hello students to solve the given question: let us use the equation of heat transfer that is equal to m c c. Here is the specific heat capacity multiplied by delta t that is, temperature difference now, using this relation? Let us solve
Numerade is a venture-backed, high-growth education technology startup based in Pasadena. We are singularly focused on creating exceptional video and interactive content experiences for education making the knowledge and skills of world class educators widely accessible and affordable to student audiences of all backgrounds. Our mission is to close the educational opportunity gap by unlocking and democratizing access to extraordinary educators and the content they have to offer.
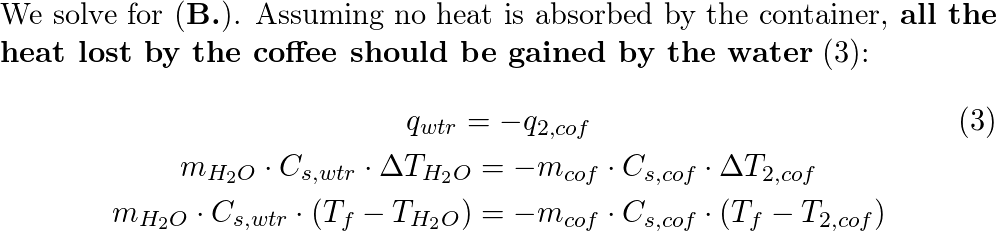
An ice cube of mass 9.0 g is added to a cup of coffee. The c

Thermo problem set no. 1
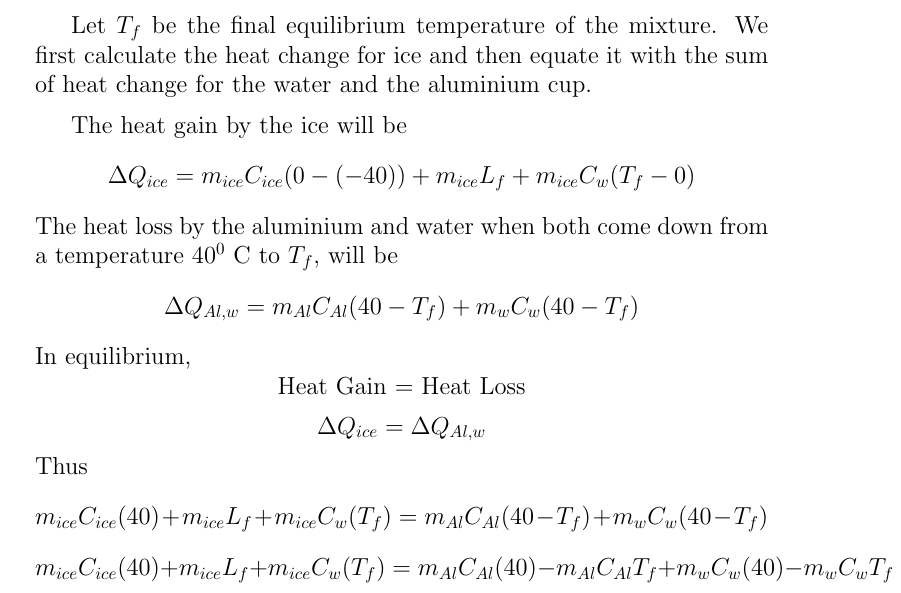
Answered: A 50 g ice cube, initially at -20degree…
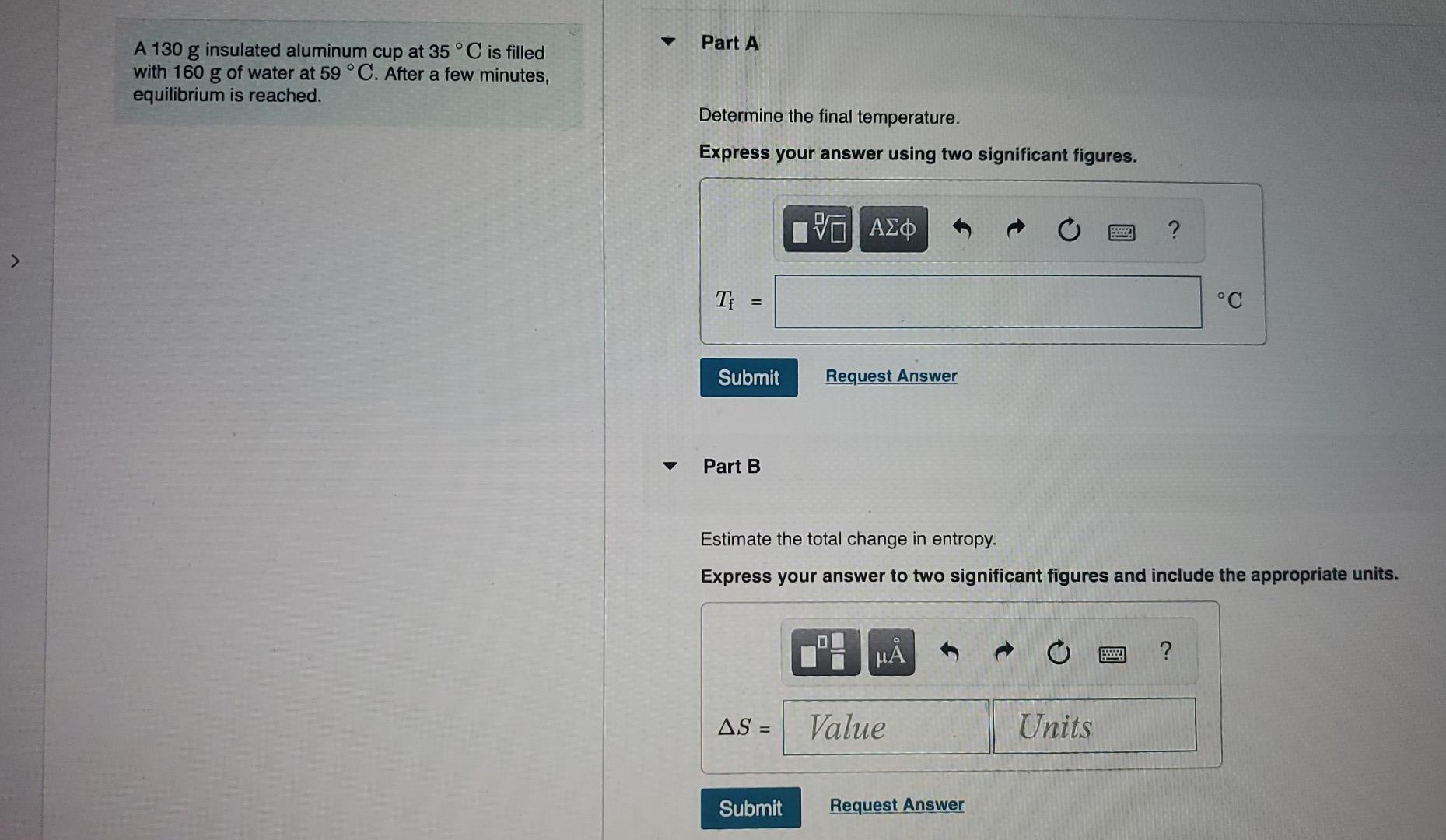
Solved Part A A 130 g insulated aluminum cup at 35 °C is
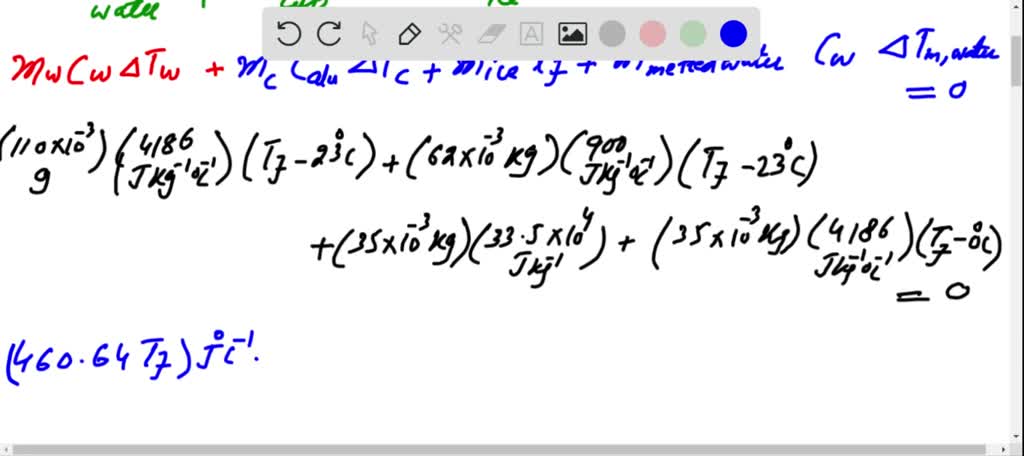
⏩SOLVED:Predict/Calculate A 35- g ice cube at 0.0^∘ C is added
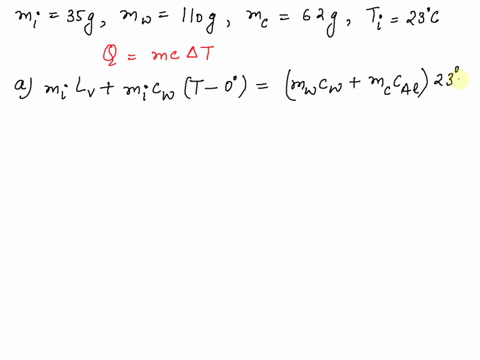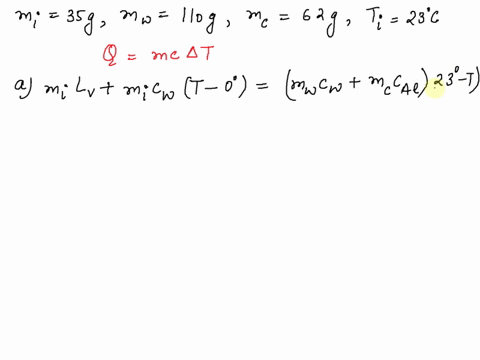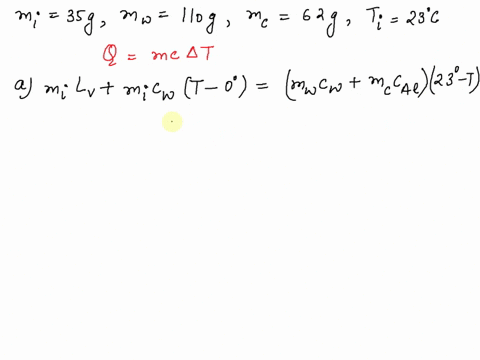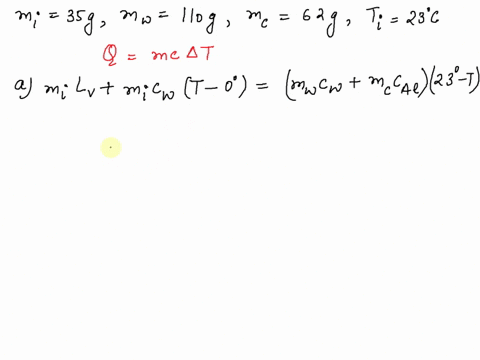
⏩SOLVED:Predict/Calculate A 35- g ice cube at 0.0^∘ C is added
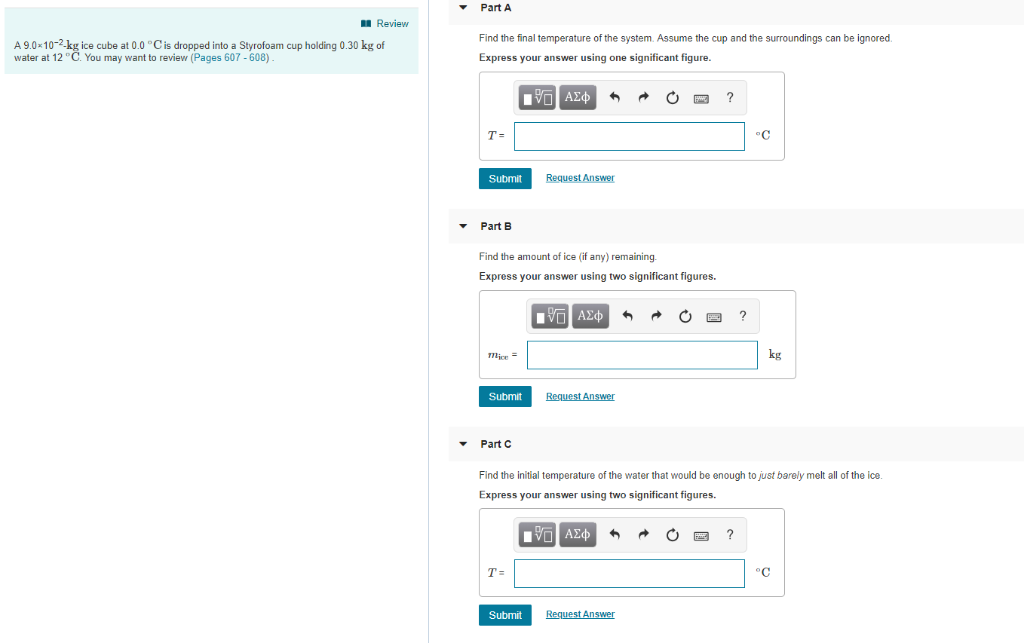
Solved A 9.0×10−2-kg ice cube at 0.0 ∘C is dropped into a
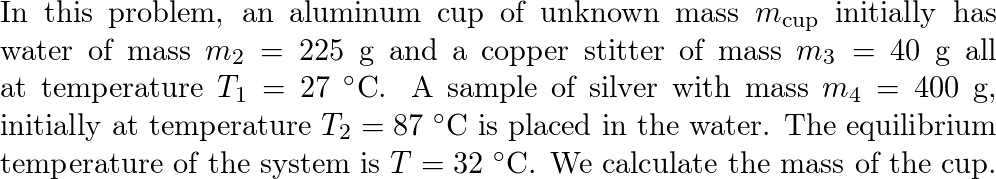
An aluminum cup contains 225 g of water and a 40-g copper st

14.24 A 0.0500-kg ice cube at −30.0ºC is placed in 0.400 kg of 35.0ºC water in a very

A.J. is drinking 500 mL (or 0.50 kg) of Dr. Pepper at 20^oC. He


⏩SOLVED: A 48 -g block of copper at -12^∘ C is added to 110 g of
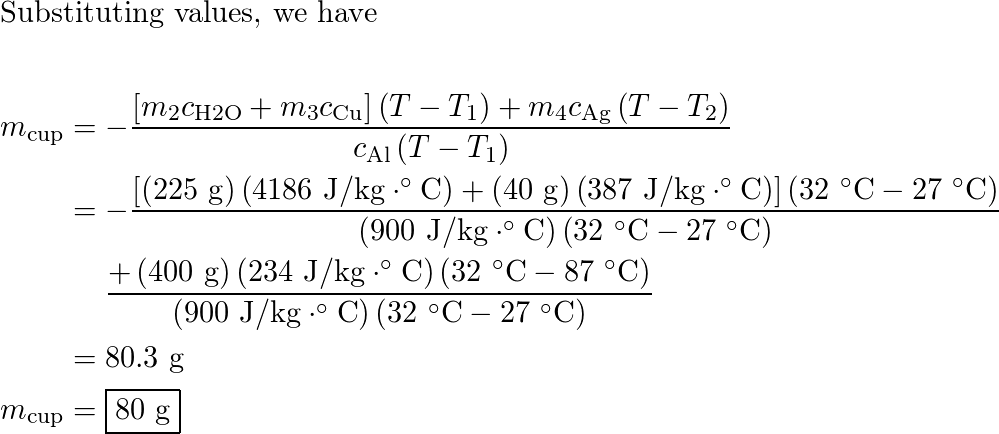
An aluminum cup contains 225 g of water and a 40-g copper st

Solved - A 153.7 g sample of ice at 0.0 °C is added to 150.7
- Blueberry Muffins [143] – Anna Olson
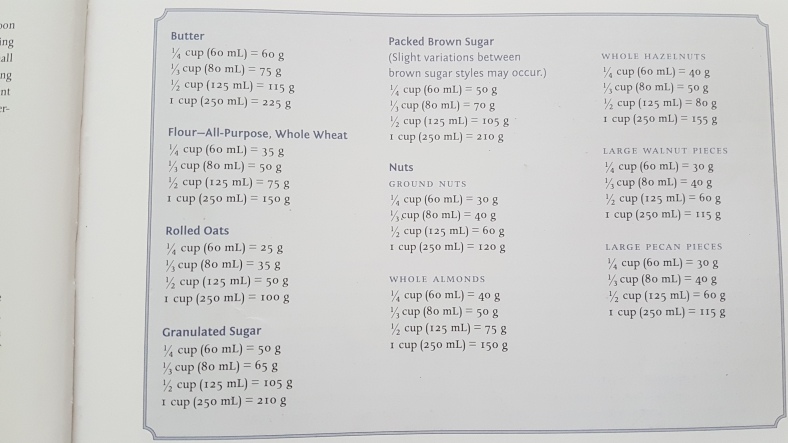
- Shockinglydelicious - Cups to gramshow many sticks of butter in 1 cuphere are your answers in an easy Conversion Chart.
- Makroclick JIMS Coffee Cup Arabica 35g*6

- ⏩SOLVED:Predict/Calculate A 35- g ice cube at 0.0^∘ C is added

- Like and save to remember how to eat high protein for fat loss if
- Hotty Hot High-Rise Skirt, Women's Skirts
- Dropship Pampers Easy Ups Training Underwear Girls Size 5 3T-4T 76

- Ladies Breifs In Prints at Rs 45/piece, Women Underwear in Mumbai

- Printed Sports Bra and High-Waisted Leggings Set – Khron Store

- Womens Anti Chafing Sweat Control Long Leg Briefs, Reduce Thigh Rubbing Mid Waist Underwear 1PK



