NGAL - Bioporto
By A Mystery Man Writer
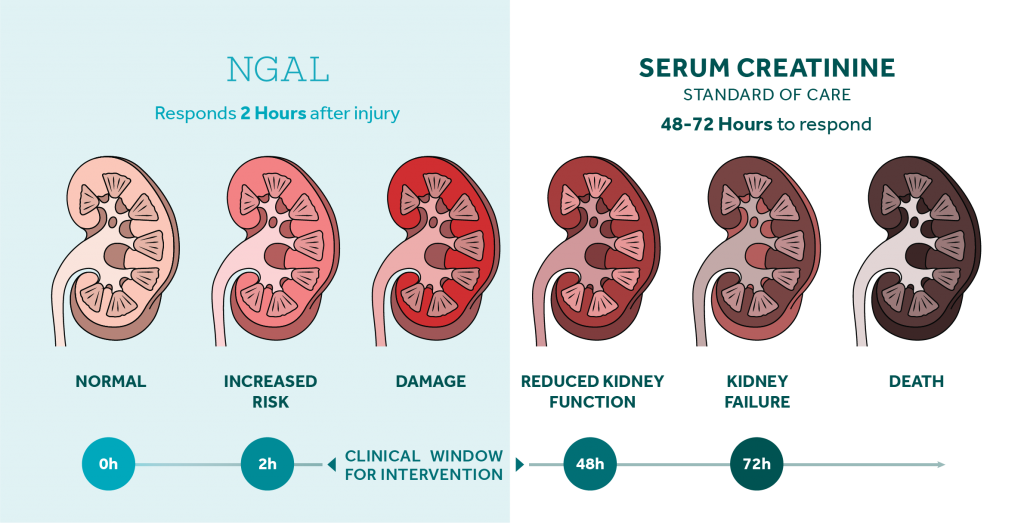
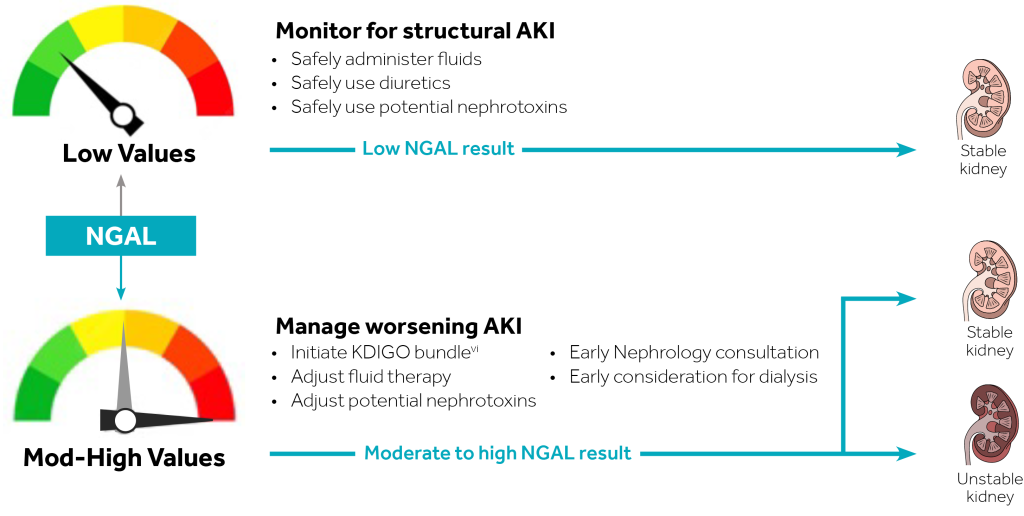
The NGAL Test is a particle-enhanced turbidimetric immunoassay for the quantitative determination of NGAL in human urine and plasma on automated clinical chemistry analyzers. NGAL measurements are useful in the risk assesment of AKI.

News - Bioporto

Identification of Urinary Activin A as a Novel Biomarker Reflecting the Severity of Acute Kidney Injury

BioPorto - Receives FDA clearance for NGAL test in the US - Inderes

The NGAL Test Reagent Kit - Bioporto
NGAL - Bioporto
BioPorto Submits Application for Marketing Authorization of NGAL Test to the US Food and Drug Administration

Elevated Neutrophil Gelatinase-Associated Lipocalin Is Associated With the Severity of Kidney Injury and Poor Prognosis of Patients With COVID-19 - ScienceDirect

Bioporto afslutter patientoptag i Ngal-studie — MedWatch

News - Bioporto

BioPorto Diagnostics A/S: Contact Details and Business Profile

About BioPorto - Bioporto

May Neutrophil Gelatinase-Associated Lipocalin (NGAL) Level Predict Mortality in Patients with Hepatocellular Carcinoma (HCC)?

Elevated Neutrophil Gelatinase-Associated Lipocalin Is Associated With the Severity of Kidney Injury and Poor Prognosis of Patients With COVID-19 - ScienceDirect
Home - Bioporto
- Plus Size T-Shirts, Women's Plus Size T-Shirts
- Kendox Pilates Wheel Training - ANCHOR DLX Orientation

- Wholesale Junior Girl's Stretch Drawstring School Uniform Joggers

- MOSHENGQI Women Ribbed Flare Leggings Seamless High Waist Bootcut Yoga Pants(S,017-Cross Wasit-Beige) : Clothing, Shoes & Jewelry

- Best deal banner. Best deal badge icon. Discount price offer. Modern vector illustration. 14435776 Vector Art at Vecteezy






