42g of N₂ react with excess of O₂ to produce NO. Amount of NO

By A Mystery Man Writer
Share your videos with friends, family, and the world

SOLVED: During the formation of ammonia, what mass of Hydrogen gas would be required to react completely with 42g of nitrogen gas?
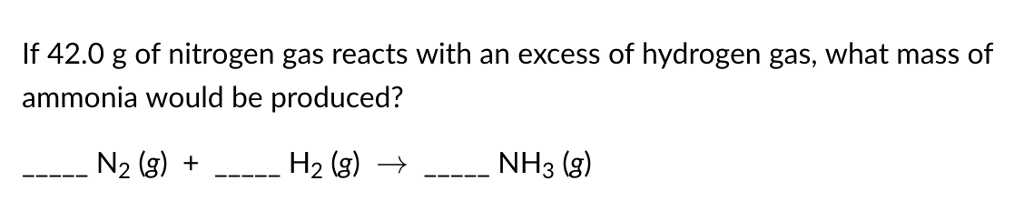
Solved If 42.0 g of nitrogen gas reacts with an excess of

UMAIR KHAN ACADEMY
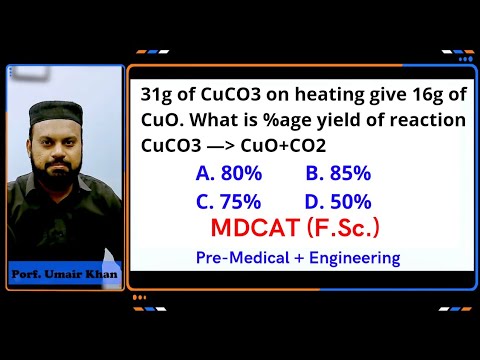
31g of CuCO3 on heating give 16g of CuO. what is %age yield of reaction. 80% 85% 75% 50%

Limiting Reaction Calculations Practice Flashcards
28g of N2 and 10g H2 are combined to form ammonia.identify limiting reagent and composition of mixture
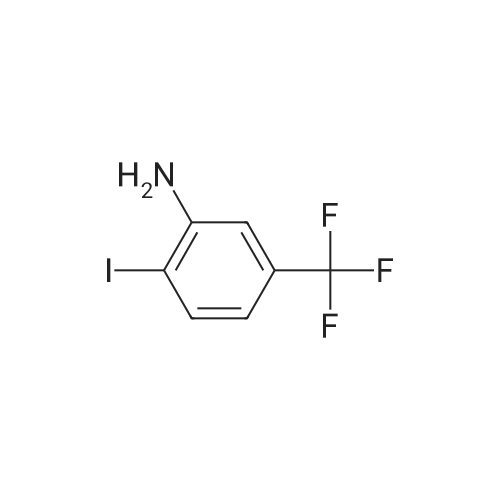
16433-96-8, 1-Ethynyl-2-nitrobenzene

stoy-key-ahm-e-tree) - ppt download

Chemistry in Daily Life Homework Help, Questions with Solutions - Kunduz

Using the balanced chemical equation: 4NH3 + 302 --> 2N2 + 6H20 Determine the amount of grams of N2 is

If 65.0 g of nitrogen dioxide is reacted with excess water, calculate the theoretical yield

Solved Identify the limiting reactant in the reaction of
- Bragg Organic Herb & Spice Seasoning – Natural Health Garden

- QTY:1), GERBER CANADA PUFFS, Fruit Taste, 8 Months+ Baby Snacks 42g, Exp2024JN05

- Machined Brass 42g Each Portal Cover (2) for Traxxas TRX-4 Scale & Trail Crawler for R/C or RC - Team Integy

- 42G Plus Size Bras by Chantelle

- Vintage C. Sebiri Sterling Silver Cuff Bracelet 42g Rare Hand Made Signed 925