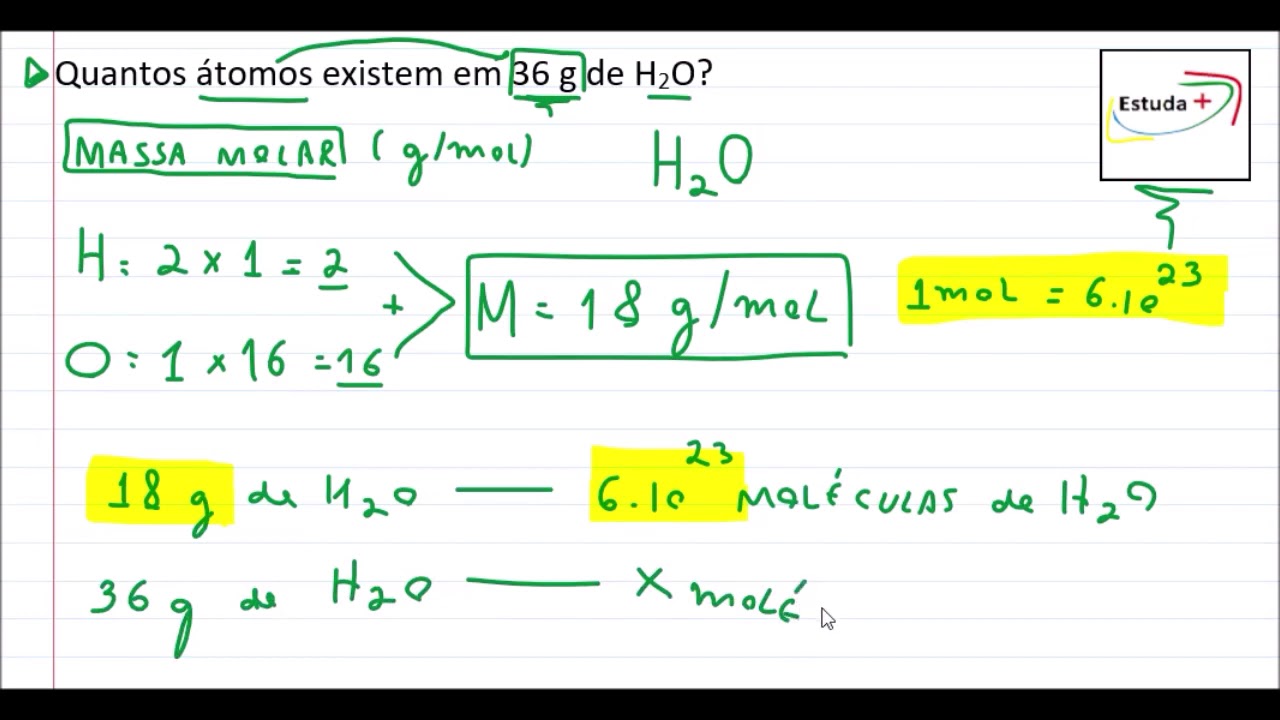
At 300 K, 36 g of glucose present per litre in its solution has an osm

By A Mystery Man Writer
pi=CRT" (C = molar concentration)" (pi(1))/(pi(2))=(C(1))/(C(2))," "(4.98)/(1.52)=(36//180)/(C(2))" or "C(2)=(36)/(180)xx(1.52)/(4.98)="0.061 M"
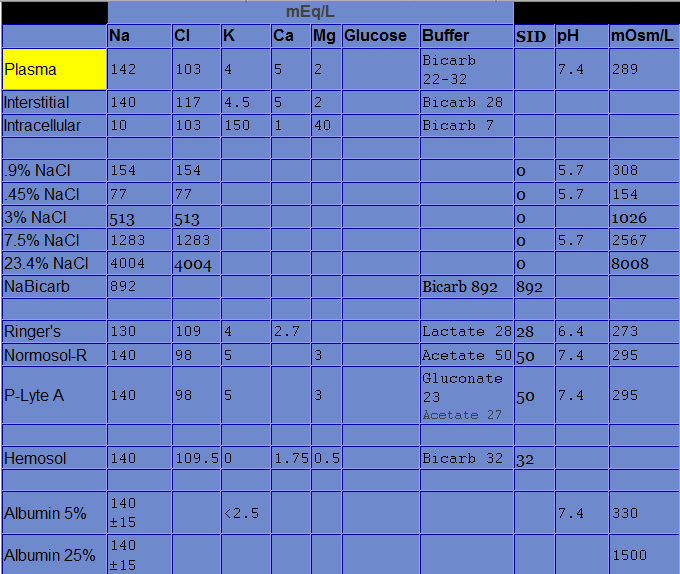
Fluid Resuscitation - Crashing Patient

The osmotic pressure of blood is 8.21 atm at 37^(@)C. How much glucose
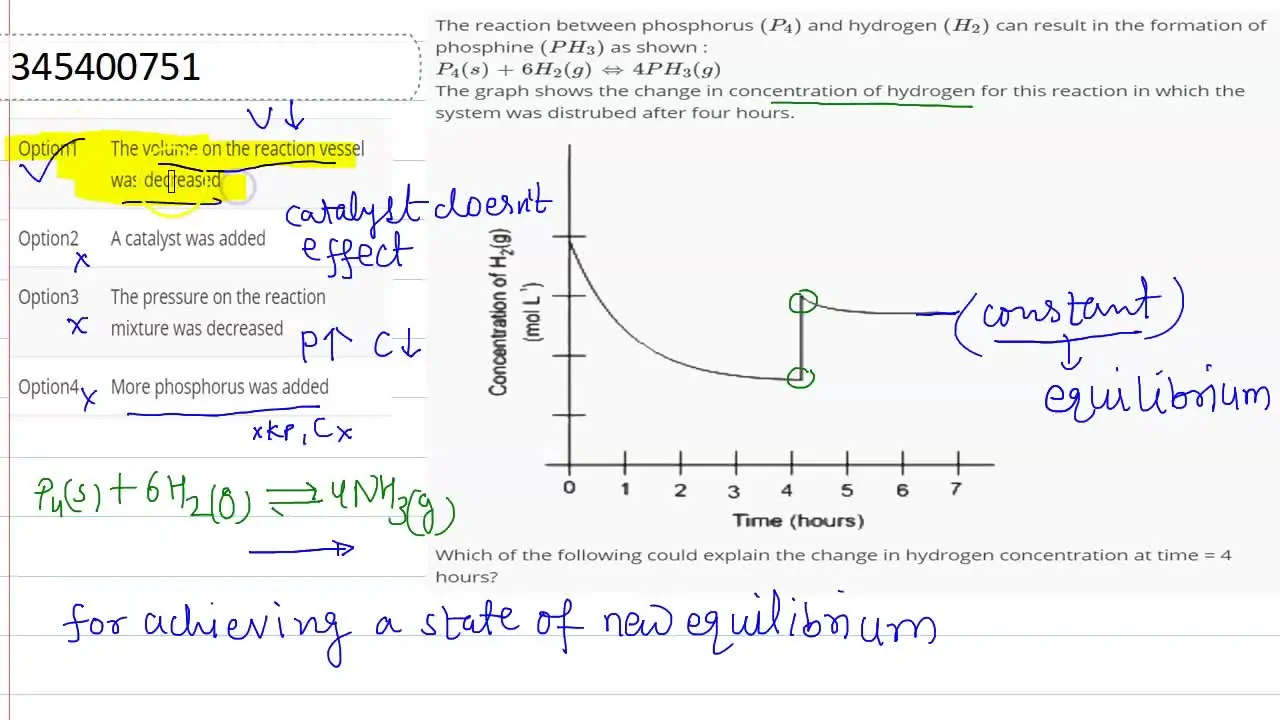
The reaction between phosphorus (P(4)) and hydrogen (H(2)) can result

Solved] At 300 K,36 g of glucose present in a litre of its solution has ..

PHRM2022 Dosage Form Design A2, PHRM2022 - Dosage Form Design A2 - UQ

An antifreeze solution is prepared from 222.6 g of ethylene glycol [C(

How many mL of a 0.1M HCl are required to react completely with 1 g mi

please explain the question and tell me what is 4 98 bar in this question and why it's not been - Chemistry - Solutions - 14451181
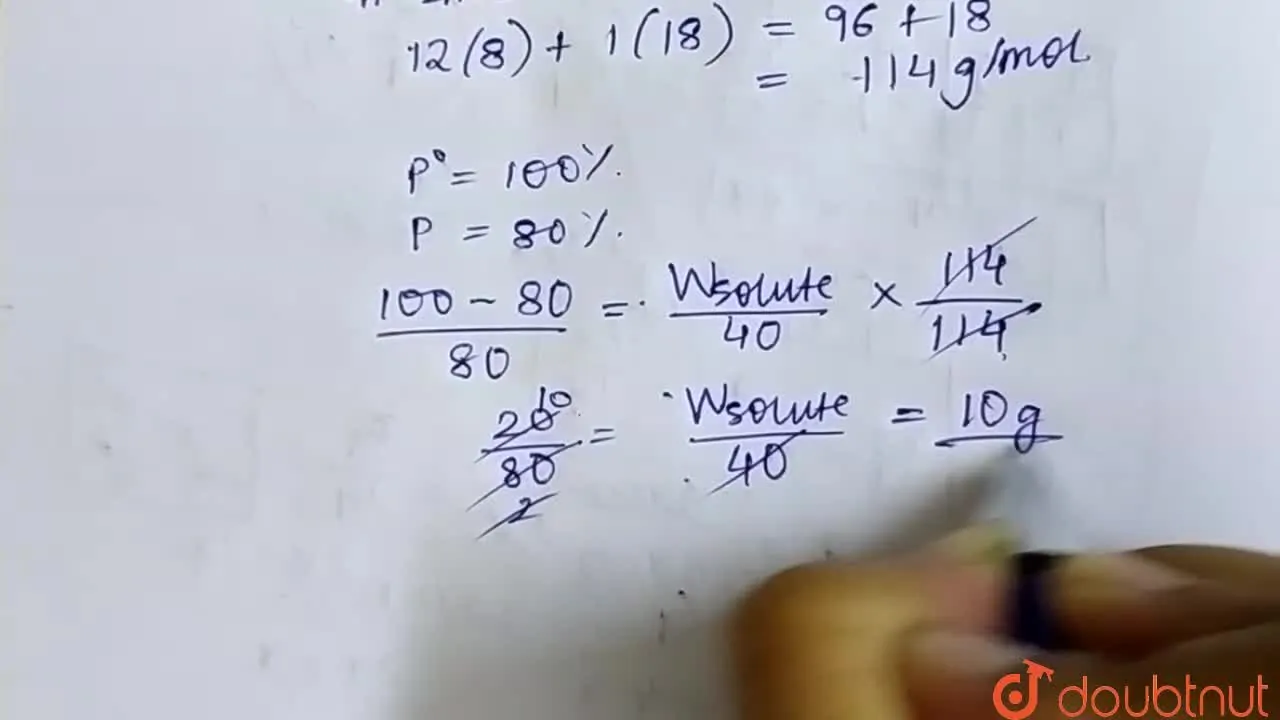
Calculate the mass of a non-volatile solute ( molecular mass 40) which

At 300 K,36 g of glucose present per litre in its solution has an osmotic..

Heyson Notes Integrated_included neurological exam (2) - Flipbook by rosalind_ip
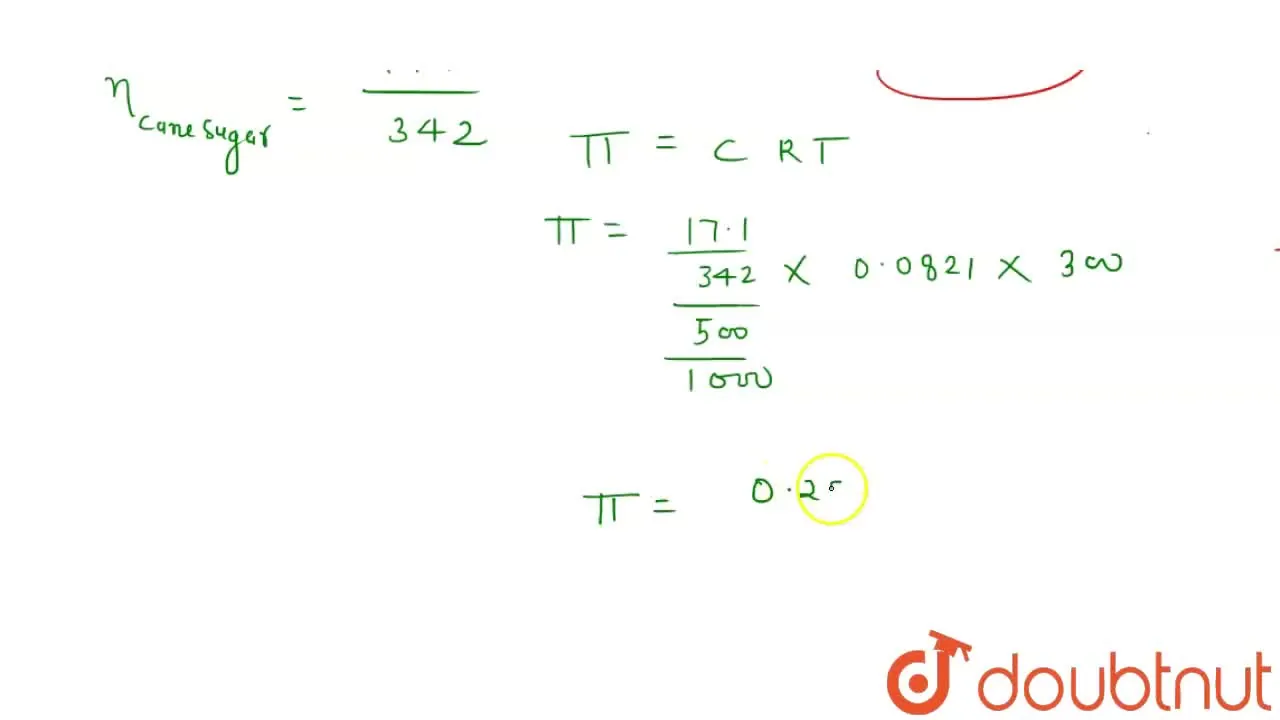
Calculate the osmotic pressure of a solution containing 17.1 g of cane

At `300 K`, `36 g` of glucose present per litre in its solution has an osmotic pressure of `4.98

Electrolytes in the ICU
- How to Choose the Right Lace for Your Wedding Dress - Pretty Happy

- Wacoal BASIC BEAUTY 853192 - Bra~vo intimates

- dailyhao Women's Cotton Underwear Mid Rise Full Coverage Soft Breathable Stretchy Solid Brief Hipster Panties for Women (5 Pack) at Women's Clothing store

- 85 Manly Must-Haves ideas quick dry pants, duluth trading, summer hats

- What Is Far Infrared Heat? Why Do Saunas Use Infrared?

- NYKD Soft Cup Easy Peasy Slip On Everyday Bra for Women, Wireless, Full Coverage, Support Shaper

- WARDROBE.NYC Cotton Cargo Pants - Farfetch

- RETURN TO FOREVER Romantic Warrior reviews

- No Bra Club shirt - Kingteeshop

- Off-White zip-front side-slit Leggings - Farfetch