How to Calculate Normality of a Solution
:max_bytes(150000):strip_icc()/how-to-calculate-normality-609580final2-0d5efa5a961f4fa0a7efc780921faee1.png)
By A Mystery Man Writer
The normality of a solution is the gram equivalent weight of a solute per liter of solution. Here are examples of how to calculate the normality.

How to Calculate Normality: 4 Steps (with Pictures) - wikiHow

SOLVED: What is the NORMALITY of a 30% w/v NaCl solution? Show your work, and answer these questions about the problem. (Note: atomic weights for Na = 22.99 and Cl = 35.45)

The normality of solution containing 31.5g of hydrated oxalic acid (H₂C₂O₄.2H₂O) - NEETLab
How to calculate the normality of liquids - Quora

Normality of NaOH solution.
calculate the normality of250 ml aqueous solution ofH2SO4 having pH=0.0

Solving dilution problems

Difference Between Molarity and Normality Definition, Units and Calculations, Relationship

Normality - Formula, Definition, Calculations [Solved Examples] - Edureify-Blog
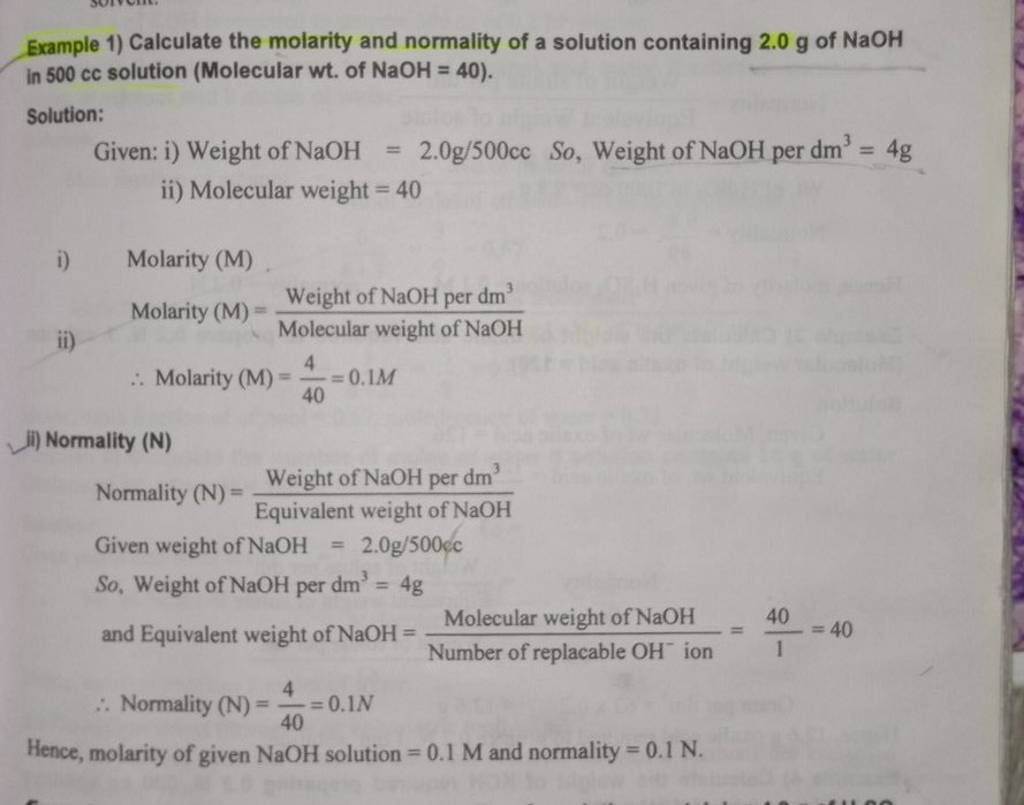
Example 1) Calculate the molarity and normality of a solution containing ..
- 36.5 degrees celsius to degrees fahrenheit - Unit Converter

- Temperature Conversion Badge Pocket Card Horizontal Accessory for Nurse Paramedic EMT for ID Badge Clip Strap or Reel
- What constitutes a fever? Fever and normal body temperature charts

- Fever Thermometer Contactless Digital Children's Forehead

- Forehead Thermometer for Adults, The Non Contact Infrared Baby
- Sarah Miller (smiller1193) - Profile

- 32D New Balance NB Dry Power Racerback High Impact Sports Bra WB71039

- Vila Ananda - O QUE É YOGA? Se você acha que yoga é apenas torcer e movimentar o corpo de forma esquisita, está na hora de repensar. Yoga é muito mais do
- Basic Over Head Hoodie
- Charlotte Russe Shop All Women's Bottoms


