How Many Molecules Are in a Drop of Water?
:max_bytes(150000):strip_icc()/GettyImages-173404960-56a1348f5f9b58b7d0bd03bd.jpg)
By A Mystery Man Writer
Learn how to calculate the number of atoms and molecules in a drop of water with this explanation.

Solved How many molecules are in each of the following? a.
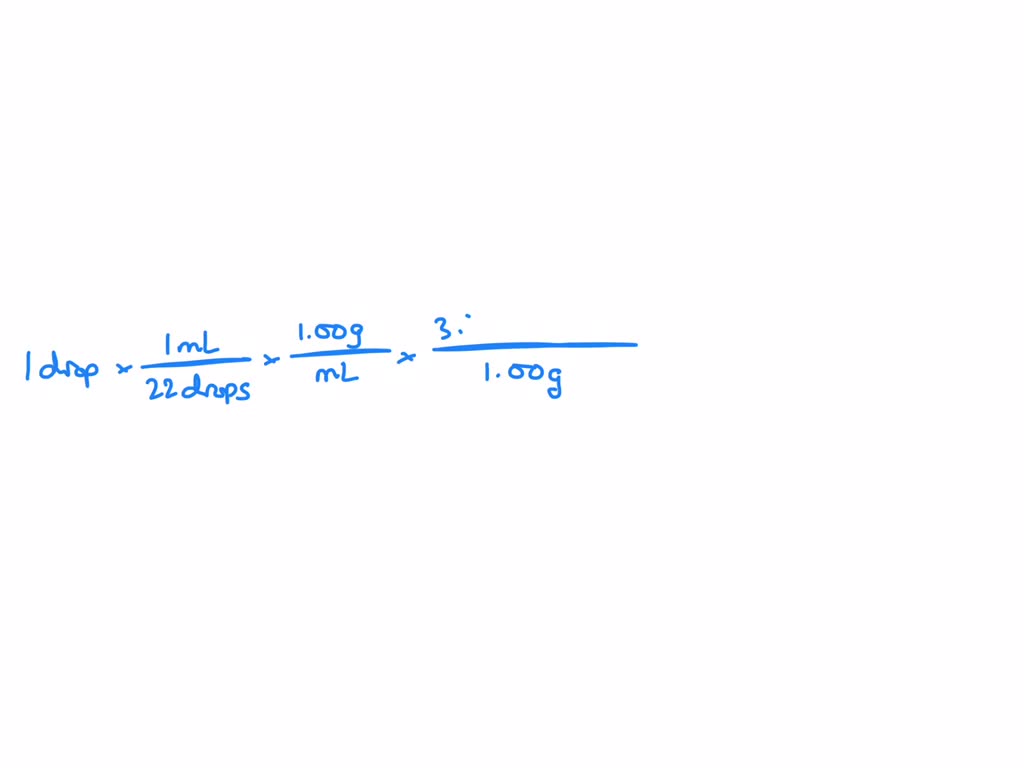
SOLVED: How many molecules of water are in one drop if there are 3.50×10^22 molecules in 1.00 g (given that 1 mL = 22 drops)? Assume that 1 mL = 22 drops

Calculate the number of molecules in a drop of water weighing 0.09 g.

How Many Atoms Are There In One Drop Of Water? 20 Most Amazing & Interesting Facts ,Part#1

How Many Molecules and Atoms in a Drop of Water?

How many atoms are there in one drop of water?

The volume of a drop of water is 0.05 mL and the density of water

Custom Rocketbook Core Executive Notebook - Full Color - Progress

What is the number of water molecules contained in a drop of water weighing `0.06 g` ?
- Best lightweight waterproof jackets in 2024 tested by experts

- HOMRAA Women's Underwire Bra, Breathable Non Padded Plus Size Full Coverage Minimizer Bras B-G Cup (Color : Beige, Size : 36C) : : Clothing, Shoes & Accessories

- Thinx For All Leaks Light Absorbency Hi-Waist Bladder Leak

- aulas-yoga-para-criancas-novidade - KR Dojo Academia

- Thoughtful House Foundation




