Graph of Compressibility Factor (Z) versus Pressure (Atm)

By A Mystery Man Writer
Graph of Compressibility Factor (Z) versus Pressure (Atm)

The given graph represents the variations in compressibility factor (z) = pV/nRT versus p, for three real gases A, B and C.Which of the following statements is/are correct?a)For gas A, a =

e Compressibility factor (Z) for hydrogen WRT pressure and temperature

Browse Free Stock Vectors and Images Created by samjore
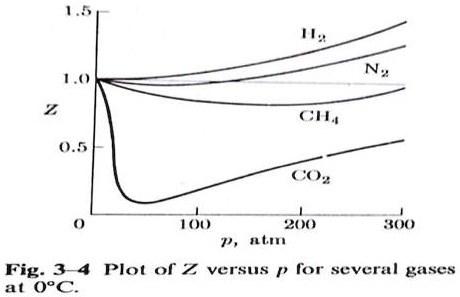
SOLVED: Subject: Compressibility Factor What is the analysis of the graph? 1.5 N 1.0 Z CHA 0.5 CO2 1 0 100 200 P, atm 300 Fig. 3-4: Plot of Z versus p for several gases at oc.
Pressure, Physics Bilder – Durchsuchen 11,170 Archivfotos, Vektorgrafiken und Videos

4.2: Real Gases (Deviations From Ideal Behavior) - Chemistry LibreTexts

Non-ideal behavior of gases (article)
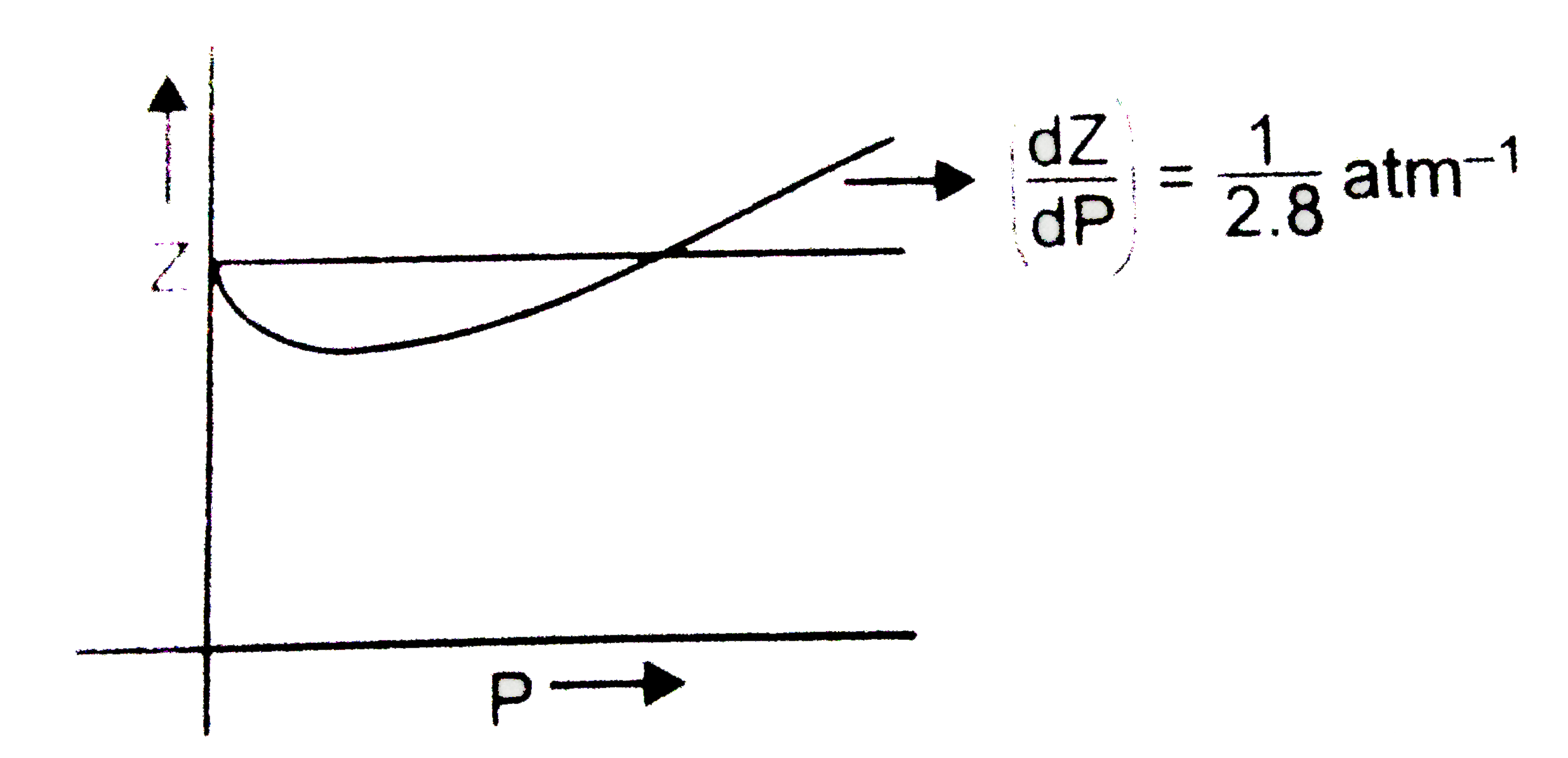
The graph of compressibility factor (Z) vs. P for one mole of a real g

Reading Compressibility Factor Charts
- Energies, Free Full-Text
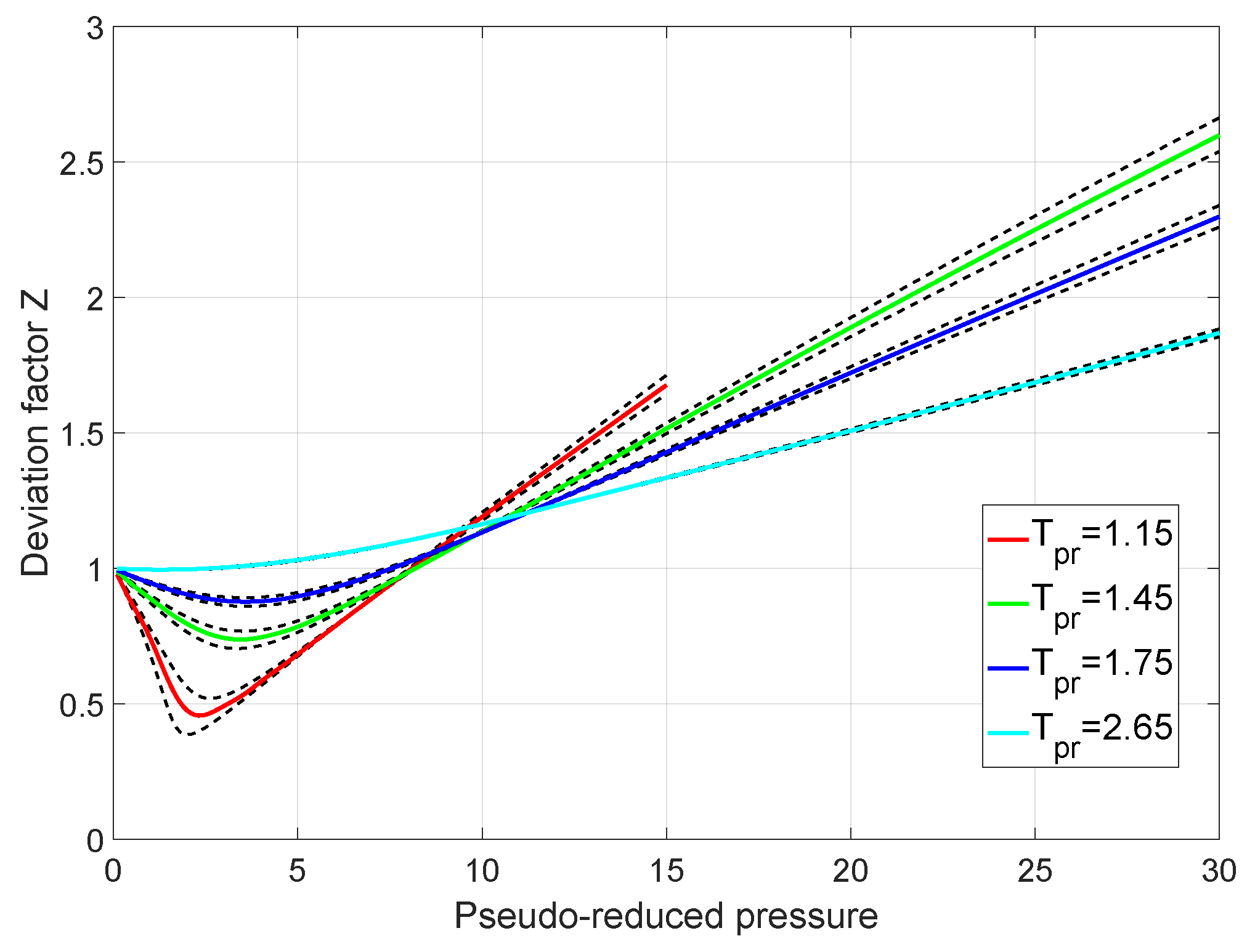
- 2024 Significance of compressibility factor - 1. What is meant by
- Oil & Gas Softwares on X: Gas Compressibility Factor Calculator (Z-Factor) New App for #iPhone and #iPad #wellcontrol #drilling #Oil and #Gas #apps at / X

- For a given gas, a graph is shown between compressibility factor (Z) and Pressure (P).Select the incorrect statement(s) about the various temperature relations.a)Temperature T1 must be above critical temperature (TC).b)Temperature T2 may

- Solved The definition of compressibility factor Z, Eq.





