Tuesday, Oct 01 2024
Five Common Mistakes Submitting a Premarket Notification

By A Mystery Man Writer
How you can avoid the most common errors made when submitting a 510(k), the “premarket notification,” with simple measures
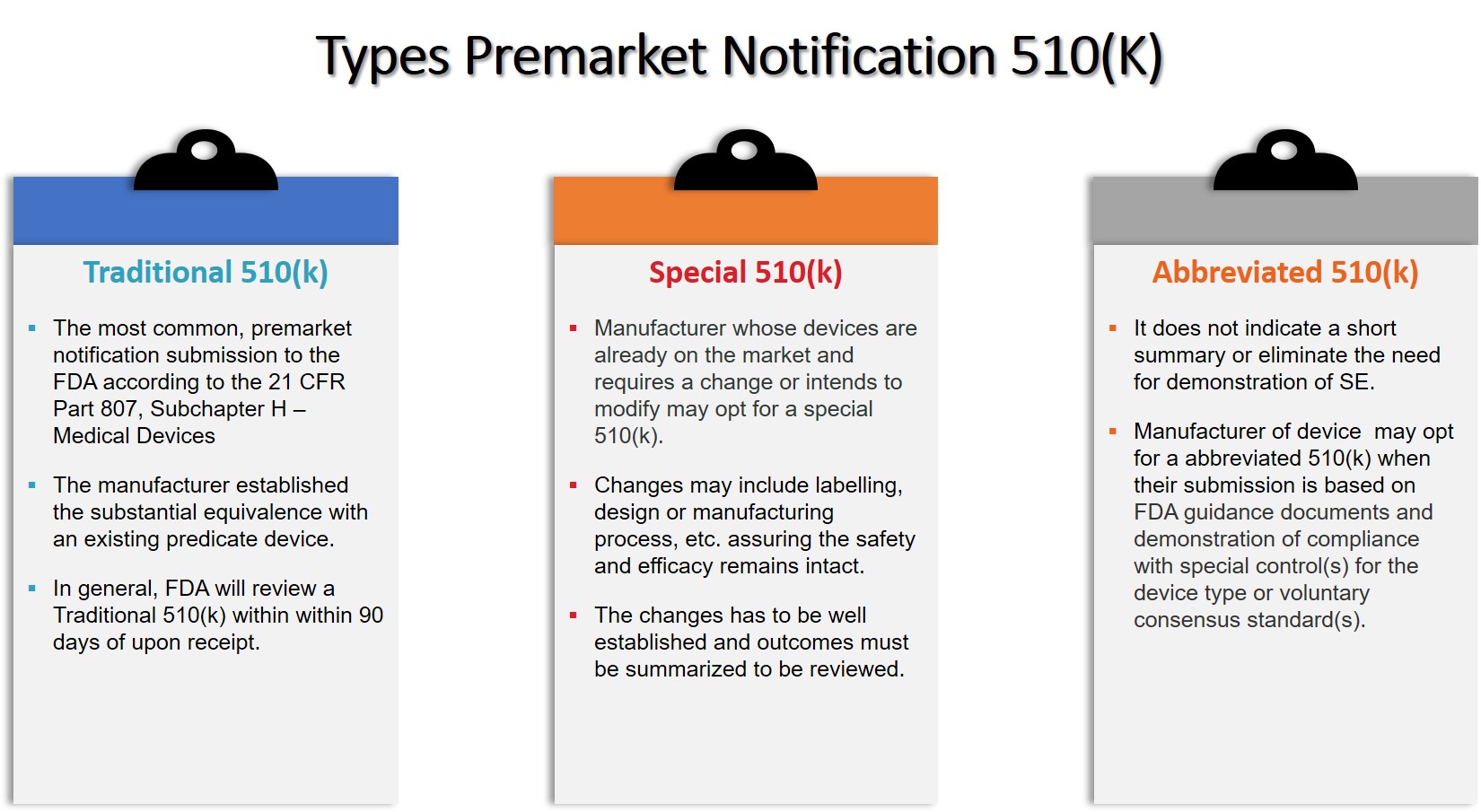
FDA 510k Premarket Notification: Essential Requirements
:max_bytes(150000):strip_icc()/us-stock-market-time-of-day-tendencies---spy-56a22dc03df78cf77272e6a2.jpg)
Common Intra-Day Stock Market Patterns

FDA 510(k) Submission: A Step-By-Step Guide On How To Prepare Yours
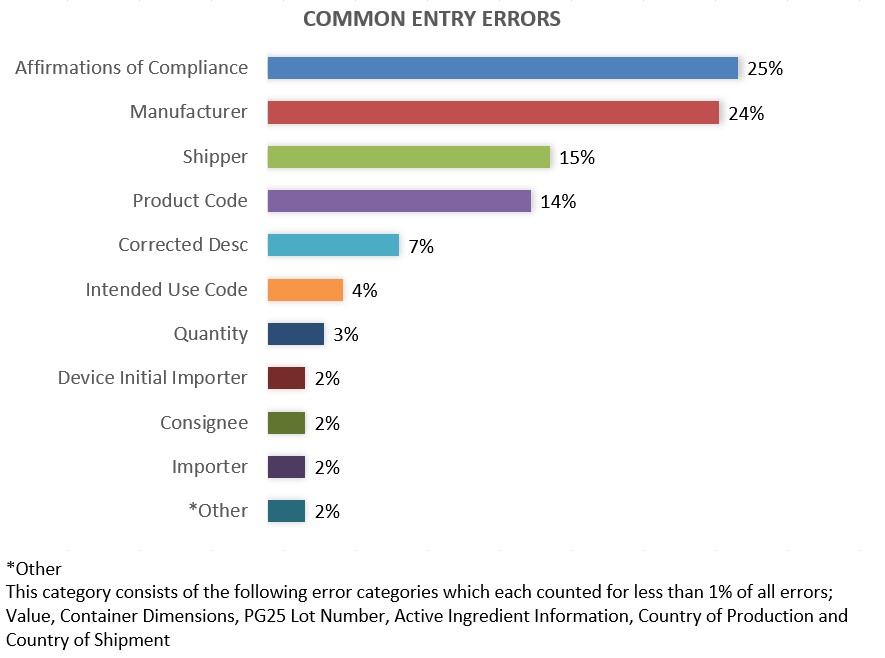
Common Entry Submission Errors

FDA Releases New Cybersecurity Premarket Guidance
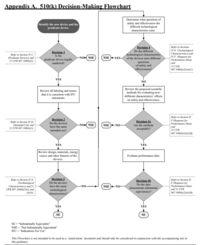
FDA: The 510(k) clearance (Premarket Notification)

Obtaining FDA 510k Clearance for Class II Medical Devices

Special Report: FDA's proposed rule to bring LDTs into the fold

Five Common Mistakes Submitting a Premarket Notification

The De Novo Classification Process A Work in Progress

510(k) Pre-Market Notification Project
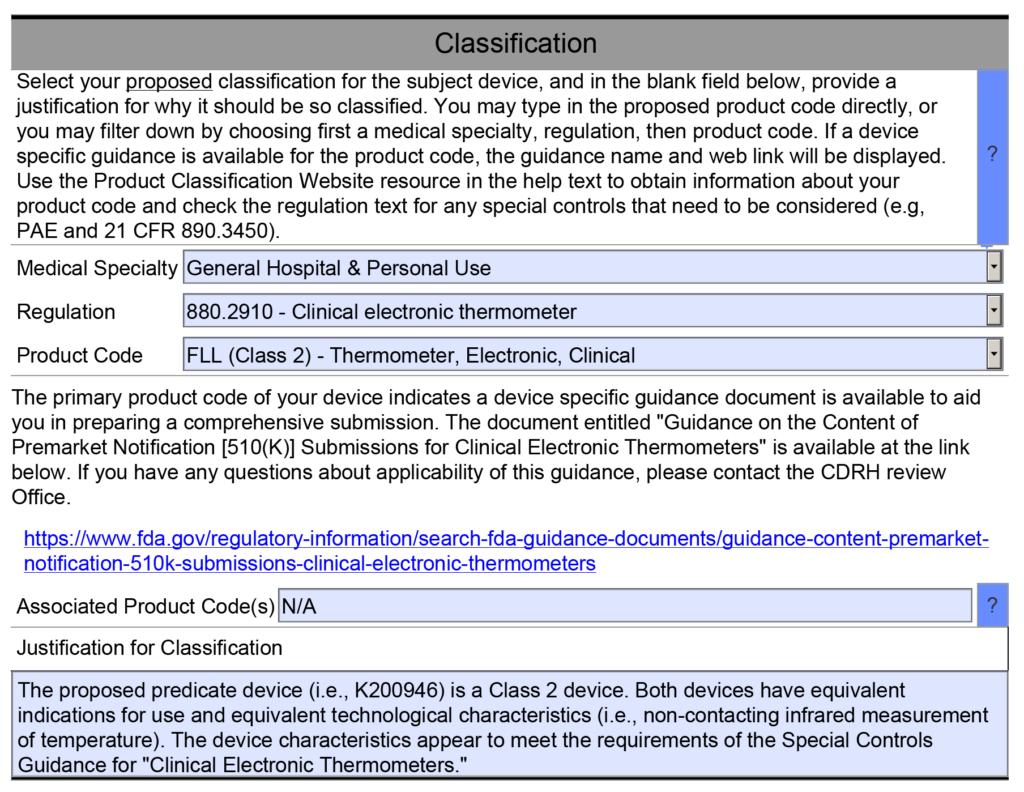
Medical Device Academy Blog Archive
Related searches
Related searches
©2016-2024, doctommy.com, Inc. or its affiliates