Sacituzumab Earns Regular FDA Approval for TNBC - NCI

By A Mystery Man Writer
Sacituzumab govitecan (Trodelvy) now has regular FDA approval for people with locally advanced or metastatic triple-negative breast cancer (TNBC), including those with brain metastases. The update follows last year’s accelerated approval of the drug for people with TNBC.

Three Treatment Modalities Emerge for Patients with Triple

FDA Approves Sacituzumab Govitecan for Triple-Negative Breast
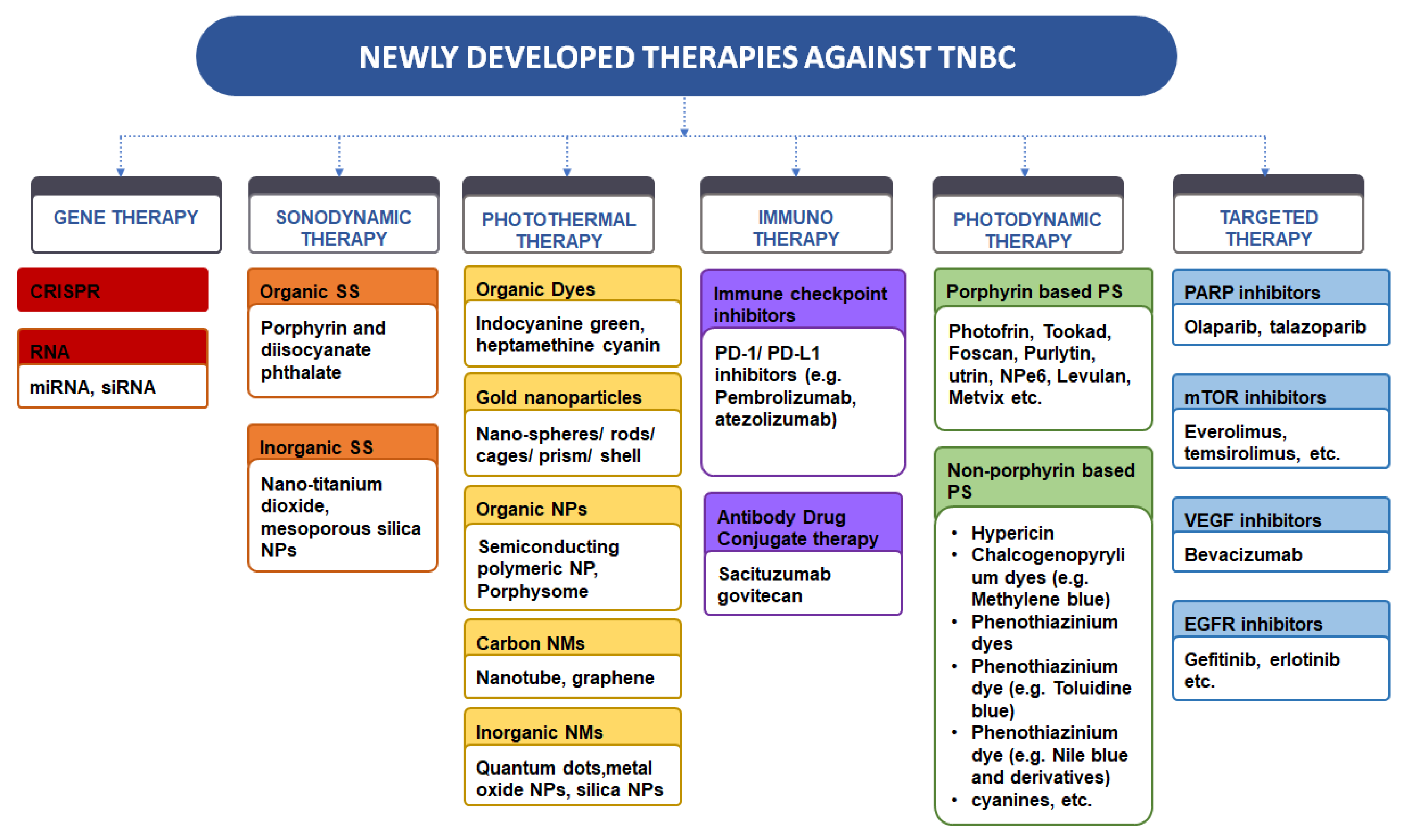
Pharmaceutics, Free Full-Text

424B3
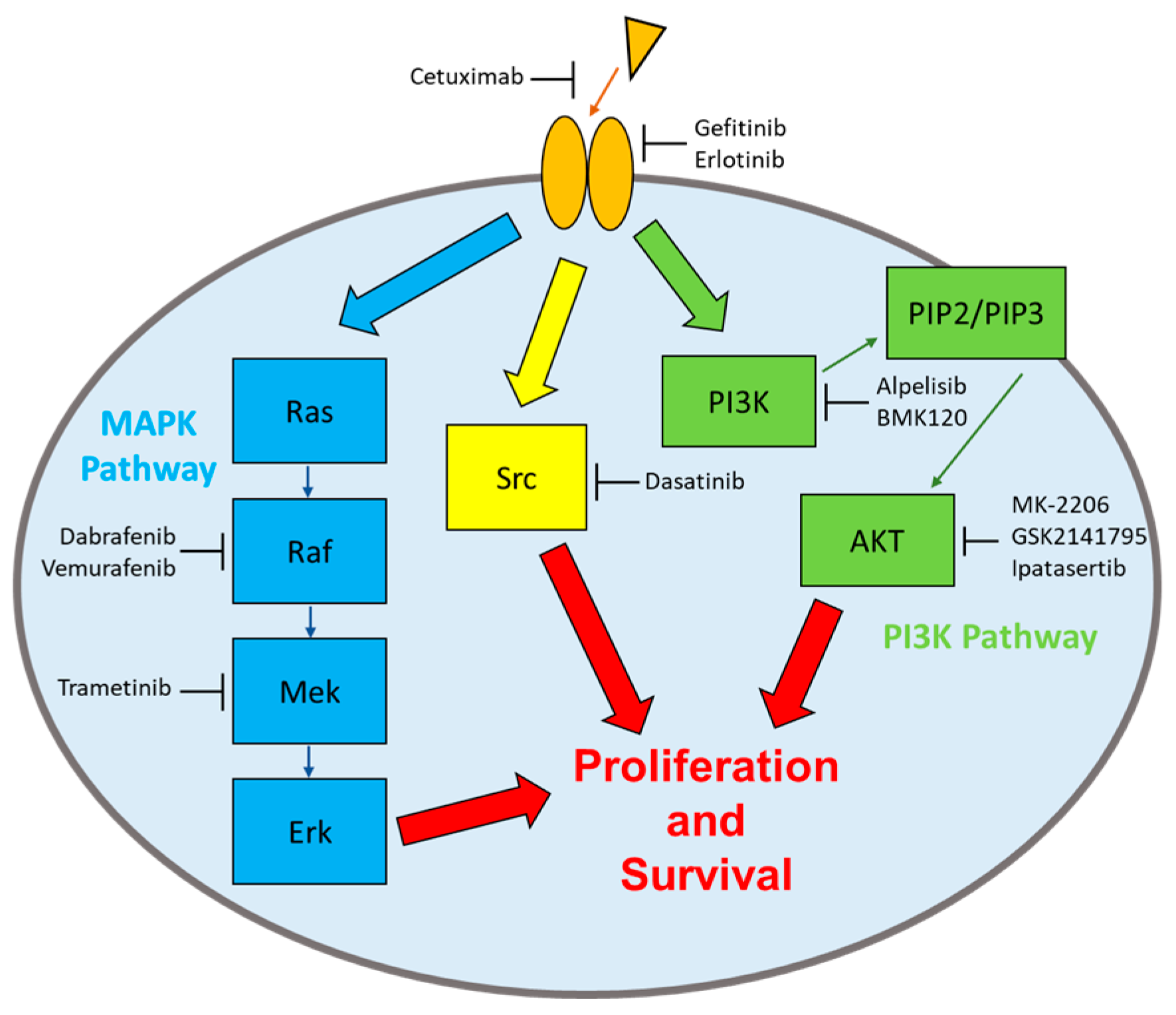
Biomolecules, Free Full-Text

Michael Weingarten (@NCISBIRdirector) / X
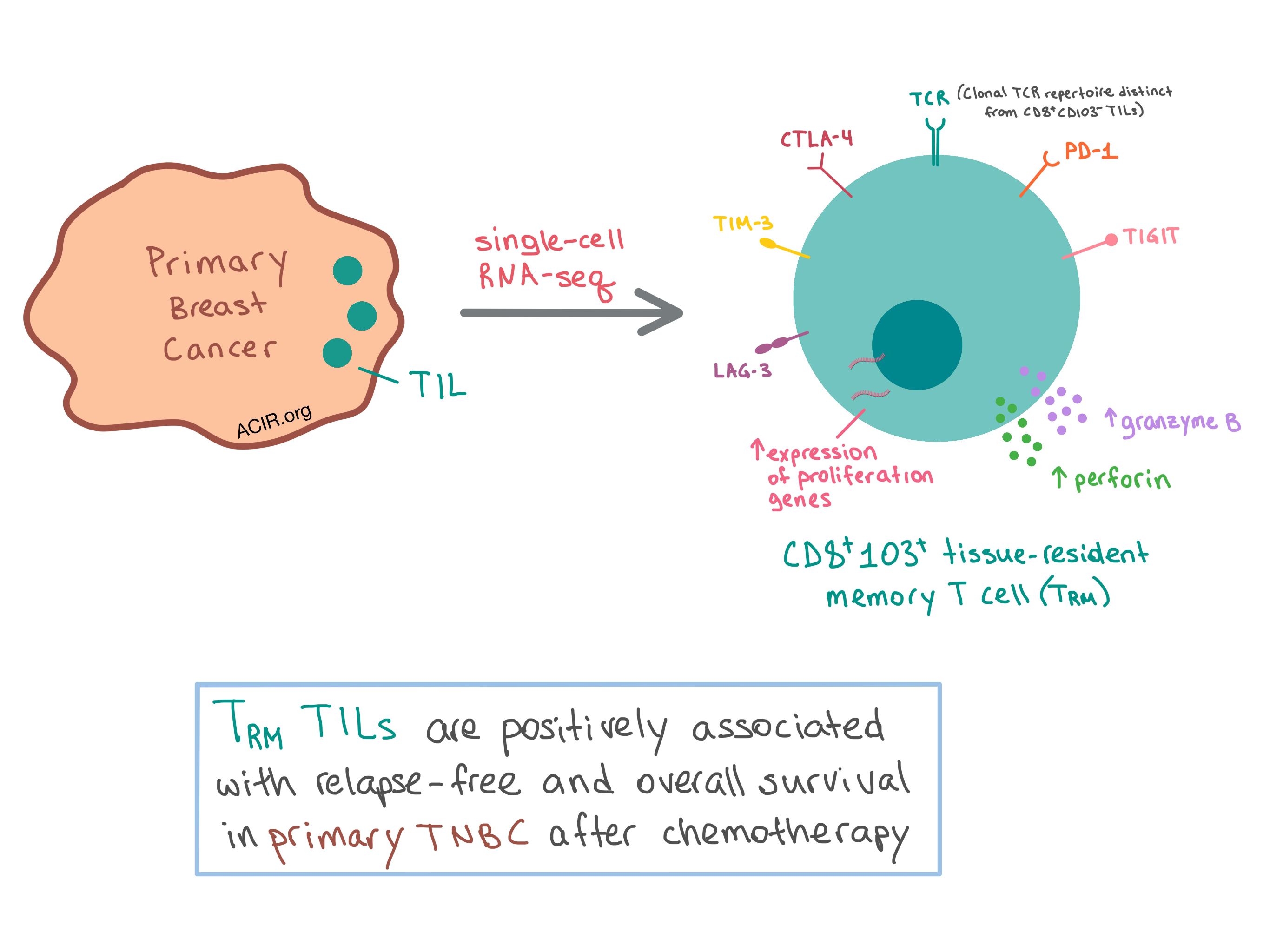
Sacituzumab Earns Regular FDA Approval For TNBC NCI
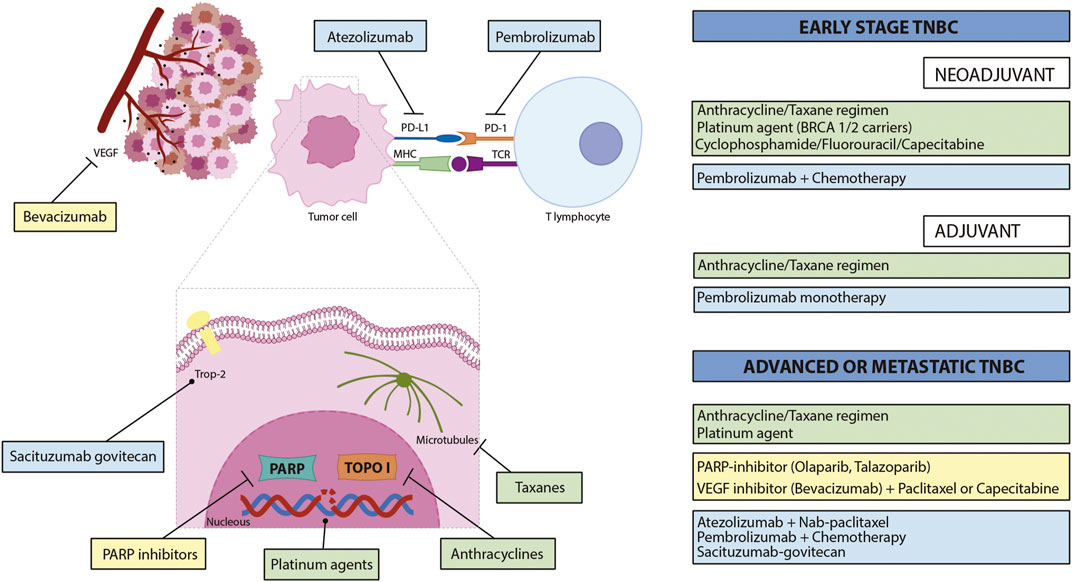
Frontiers Immunotherapy in triple-negative breast cancer

Therapeutic efficacy of IMMU-132 with different DARs. NCI-N87

Sacituzumab Earns Regular FDA Approval for TNBC - NCI

Sacituzumab Earns Regular FDA Approval For TNBC NCI
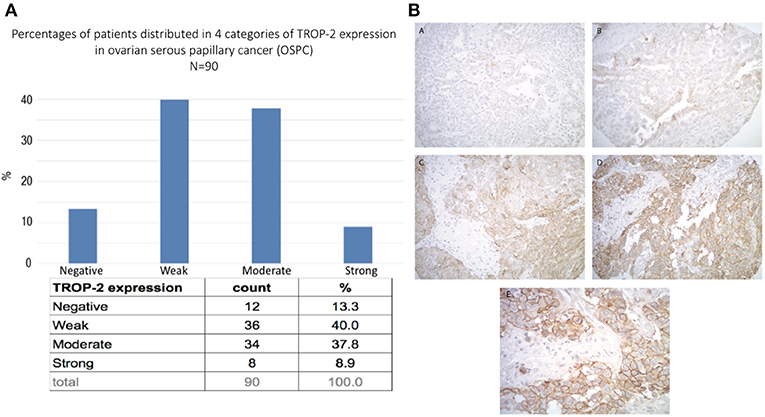
Frontiers Preclinical Activity of Sacituzumab Govitecan, an Antibody-Drug Conjugate Targeting Trophoblast Cell-Surface Antigen 2 (Trop-2) Linked to the Active Metabolite of Irinotecan (SN-38), in Ovarian Cancer
.jpg)
New FDA Alert Warns of Drug Combination for Advanced Triple-Negative Breast Cancer Patients

Sacituzumab govitecan in previously treated hormone receptor

Triple negative breast cancer: Pitfalls and progress
- New Sexy Lingerie Bra Push Up Lace 32 34 36 38 40 42 44 A B C D E Elastic Bras Women Plunge Embroidery Underwire Lace Bralette

- Salmo 38 - A Malignidade do Pecado - Segunda Igreja Batista em Goiânia

- 38 Super: The Semi-Auto .38 That's Slipping Into Obscurity

- 38 Special vs 9mm - Difference and Comparison

- Lockheed P-38L Lightning > National Museum of the United States Air Force™ > Display





