Ideal Gas Law Equation Compressibility Of Natural Gas - Chemistry
By A Mystery Man Writer
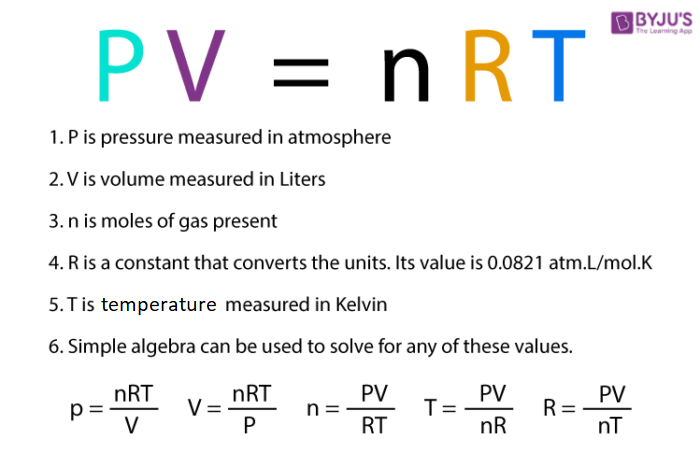
Ideal Gas Law Equation calculator solving for pressure given moles, universal Gas constant, temperature and volume. Learn the behavior of ideal Gases, factors affecting them and the laws obeyed by ideal Gases. Join Byju
Ideal Gas Law Equation calculator solving for pressure given moles, universal Gas constant, temperature and volume. Learn the behavior of ideal Gases, factors affecting them and the laws obeyed by ideal Gases. Join Byju's for Learning more concepts.

Real Gases vs Ideal Gases & the Compressibility Factor

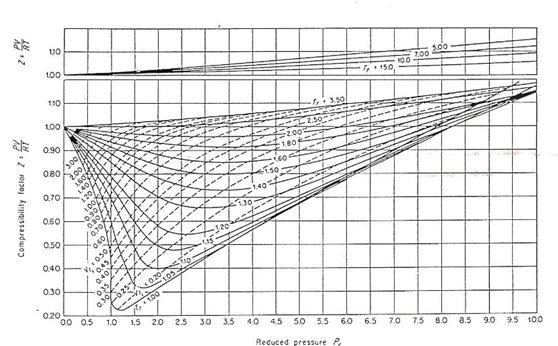
Real gasses For an ideal gas, the compressibility factor Z = PV/nRT is equal to unity for all conditions. For a real gas, Z can be expressed as a function. - ppt

Compressibility Factor Z Important Concepts and Tips for JEE Main
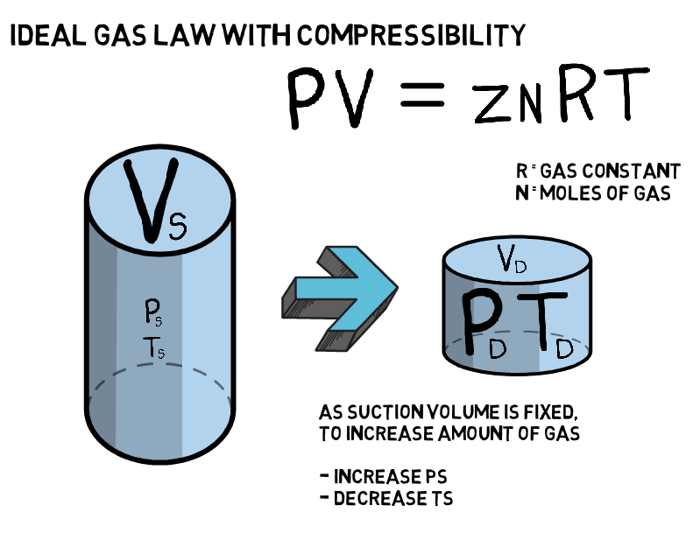
Enalysis Tip 1.17: Natural Gas Compression Basics 1 - Gas Properties

4.2: Real Gases (Deviations From Ideal Behavior) - Chemistry LibreTexts
What is the value of compressibility factor in terms of vander waal cons†an t at different conditions of pressure and volume?Why is Z>1 for H2 and He gas
How the ideal gas law helped us creating a software tool called

Compressibility Factor Calculator - File Exchange - MATLAB Central
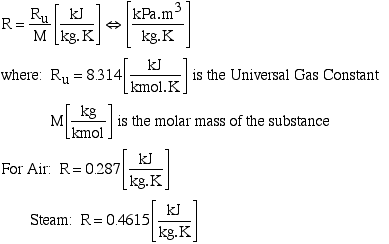
Chapter 2b: Pure Substances: Ideal Gas (updated 1/17/11)

1 Equations of State The relationship among the state variables, temperature, pressure, and specific volume is called the equation of state. We now consider. - ppt download

Ideal Gas - an overview
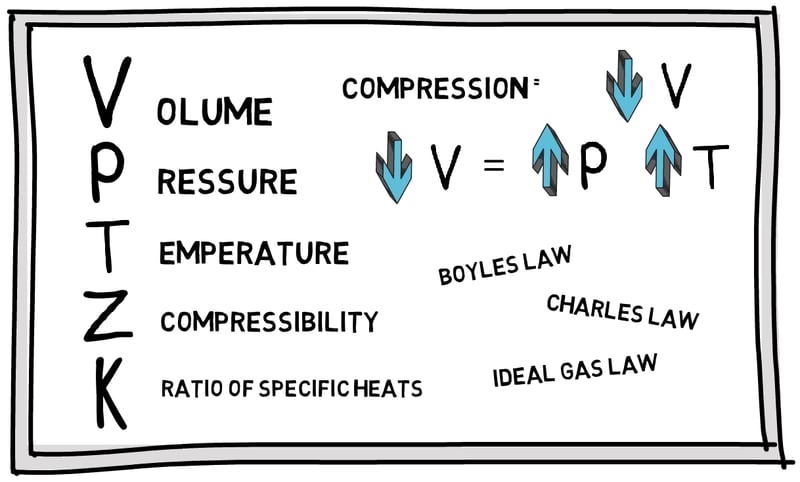
Enalysis Tip 1.16: Natural Gas Compression Basics – An Introduction

Real Gas Behavior The Compression Factor (Z) [Example #2]
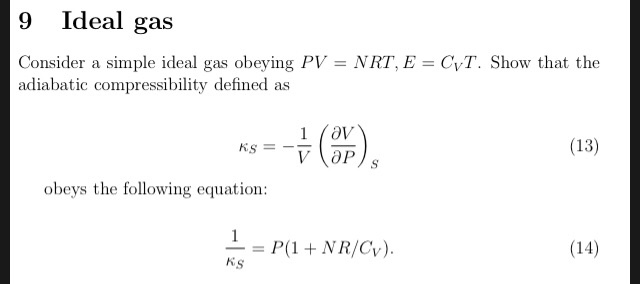
Solved 9 Ideal gas Consider a simple ideal gas obeying PV








