Thermal Energy & Heat. What is Temperature? Temperature measure
By A Mystery Man Writer
Temperature Conversions o C to o F: o F = 9/5( o C) + 32 o F to o C: o C = 5/9( o F – 32) o C to K: K = o C K to o C: o C = K – 273
What is Temperature Temperature measure of the average KE of all the particles within an object
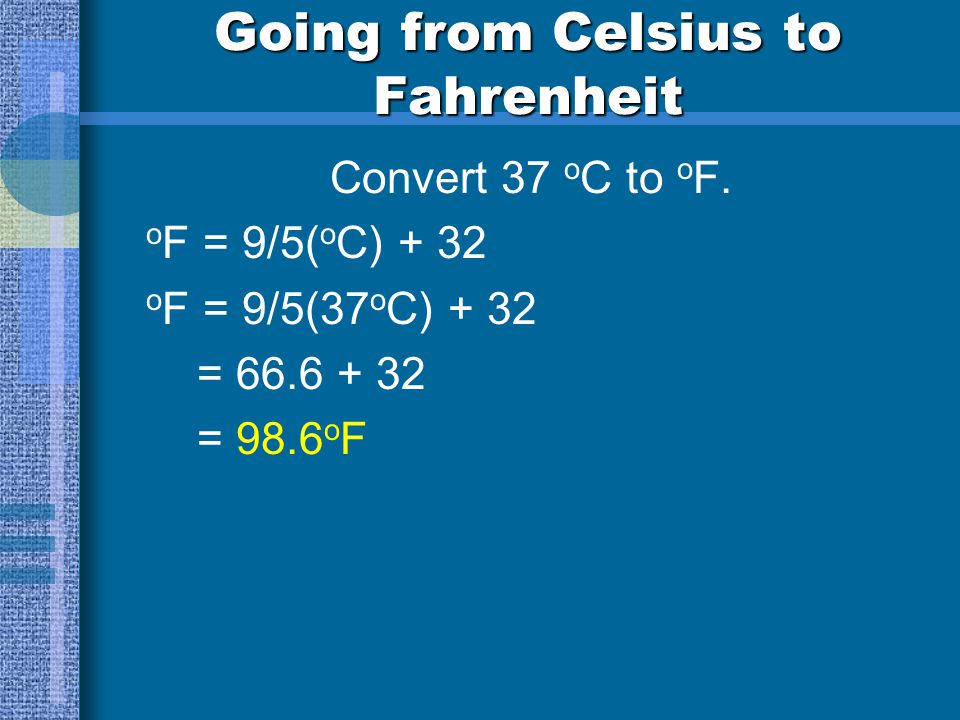
o F = 9/5( o C) + 32 o F = 9/5(37 o C) + 32 = = 98.6 o F.
Going from Fahrenheit to Celsius Convert 68 o F to o C o C = 5/9( o F – 32) o C = 5/9(68 – 32) = 5/9(36) = 20 o C
Going from Celsius to Kelvin Convert 100 o C to K K = o C K = = 373 K
Going from Kelvin to Celsius Convert 310 K to o C o C = K – 273 o C = 310 – 273 = 37 o C
Thermal Energy the total energy of the particles in a material KE - movement of particles PE - forces within or between particles due to position depends on temperature, mass, and type of substance
B - same temperature, more mass 200 mL 80ºC A 400 mL 80ºC B.
measured in joules (J) a transfer of energy.
80ºC A 10ºC B Heat flows from A to your hand = hot. Heat flows from your hand to B = cold..
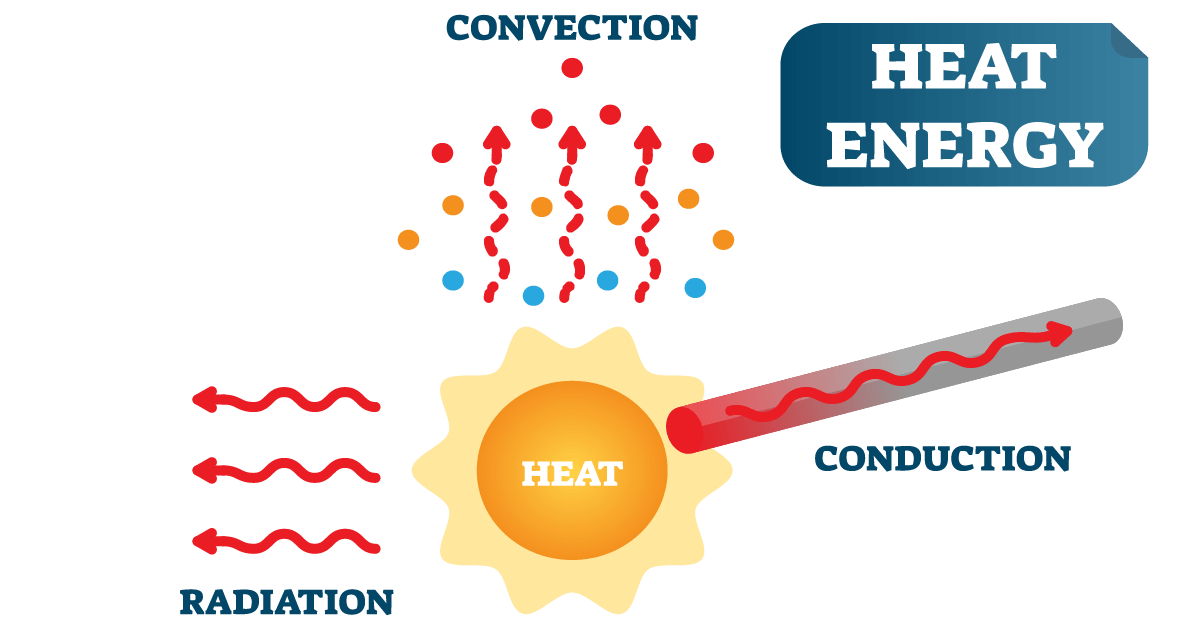
Insulators slow the transfer of heat due to air pockets. Conductors easily allow the transfer of heat, like metals. Heat is transferred by conduction, convection, and radiation..
Occurs best in solids. Heat continues to be transferred until both objects reach the same temperature, called a thermal equilibrium..
The cause of wind and weather..
Transferred in all directions. No contact required. Dark or dull objects absorb more than light or shiny objects do..
of 1 kg of material by 1 degree Kelvin units: J/(kg·K) or J/(g·°C).
50 g Al50 g Cu Al - It has a higher specific heat. Al will also take longer to cool down..
Heat Transfer Q = m T C p Q:heat (J) m:mass (g) T:change in temperature (K or °C) C p :specific heat (J/g·K or J/g.o C) T = T f - T i – Q = heat loss + Q = heat gain
Heat Transfer Calorimeter device used to measure changes in thermal energy Coffee cup Calorimeter in an insulated system, heat gained = heat lost
How much heat is lost by the spoon. GIVEN: m = 32 g T i = 60°C T f = 20°C Q = . C p = 235 J/kg·K WORK: Q = m· T·C p m = 32 g = kg T = 20°C - 60°C = – 40°C T = 293 K – 333 K = -40 K Q = (0.032kg)(-40 K)(235J/kg·K) Q = J (lost heat, negative).
GIVEN: m = 230 g T i = 12°C T f = 90°C Q = . C p = J/g· o C WORK: Q = m· T·C p m = 230 g T = 90°C - 12°C = 78°C Q = (230 g)(78 o C)(4.184 J/g· o C) Q = 75,061 J (gained heat, positive).

Thermal Energy from Light
What is conductive heat transfer?

Heat Loss and Gain Heat Loss and Gain - ppt video online download

SOLUTION: Heat and temperature - Studypool
Thermal Energy Calculator - Calculator Academy
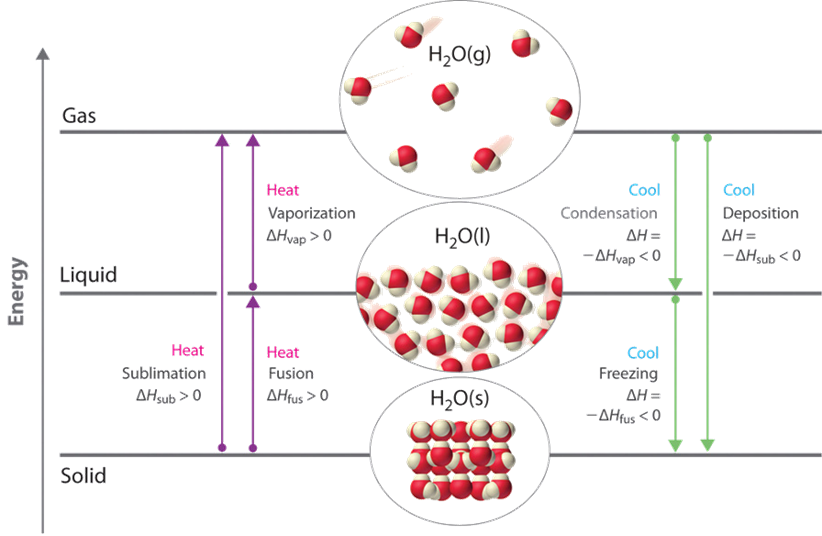
1.9: Heat and changes in physical states of matter - Chemistry LibreTexts

Temperature and Heat: Introduction, Types, Videos and Solved Examples
:max_bytes(150000):strip_icc()/heat-energy-definition-and-examples-2698981-final-2-5b76efbcc9e77c005028d736.png)
Definition and Examples of Heat Energy

DOC) Subject: PHYSICS Topic: HEAT ENERGY 2 (TEMPERATURE & ITS MEASUREMENTS) Class: SS2

Thermal Energy & Heat. What is Temperature? Temperature measure of the average KE of all the particles within an object. - ppt download

Guided Notes: Heat & Temperature - ppt download
Calorimetry General Chemistry 101/102 Laboratory Manual University of North Carolina at Wilmington. - ppt download

Phyjits Tutorials - Thermal Energy or Heat is a topic in IGCSE Physics 0625 that covers the detailed understanding of heat and how heat as an energy is transferred. Temperature in general
- Europe heat wave by the numbers: Record-breaking temperatures blasted France, Belgium, Germany, Netherlands, Britain

- Climate of Spain - Wikipedia
- Thermochemically driven crystal phase transfer via chlorination roasting toward the selective extraction of lithium from spent LiNi1/3Co1/3Mn1/3O2 - ScienceDirect

- What temperature is 36.5 Celsius? - Quora
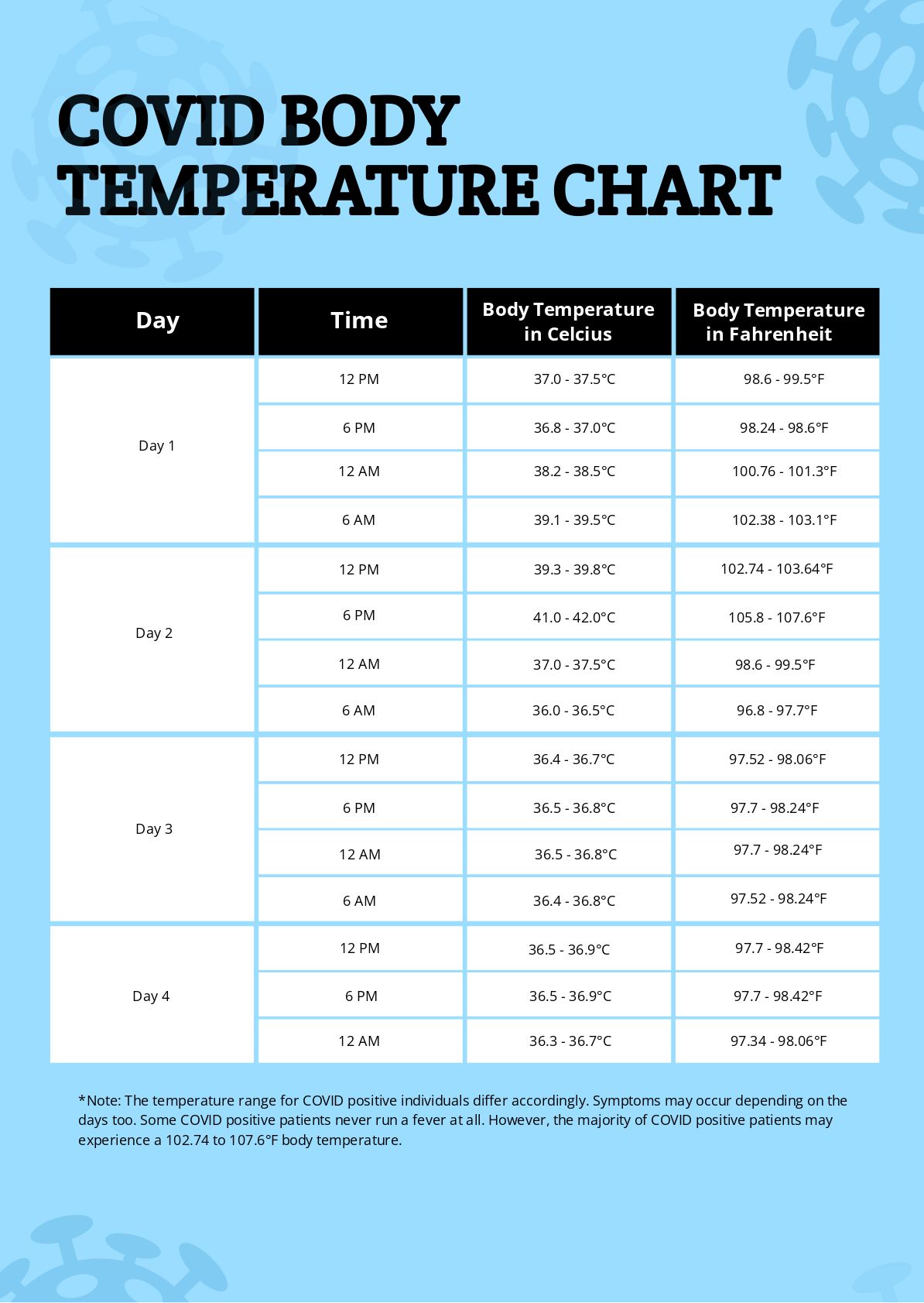
- Covid Body Temperature Chart in PDF - Download