Compressibility factor (Z) for a van der Waals real gas at

By A Mystery Man Writer
Share your videos with friends, family and the world

image.slidesharecdn.com/unit10realgasesvdwfl14fina

Isenthalpic point and real gas compressibility relation

The compressibility factor a real gas high pressure is:-1 - frac{Pb} {RT}1 + frac {RT} {Pb}11 + frac {Pb} {RT}
6.3: Van der Waals and Other Gases - Physics LibreTexts

5.6 Non-Ideal Gas Behavior – Chemistry

2.8 – Real/Non-Ideal Gas Behaviours – General Chemistry for Gee-Gees

Deviation of Gas from Ideal Behavior
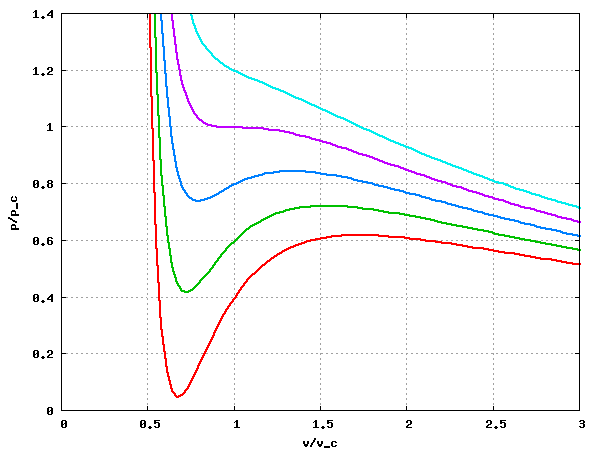
For a certain van der Waal's gas, critical temperature is-243^(@)C. Ma
Complete Solutions to Mock Test 1 of chapter MOCK TEST of Class 11 book with complete answers and questions
Van der Waals equation of state page on SklogWiki - a wiki for statistical mechanics and thermodynamics

Answered: Compression factor of a gas with van…
For a certain van der Waal's gas, critical temperature is-243^(@)C. Ma
Complete Solutions to Mock Test 1 of chapter MOCK TEST of Class 11 book with complete answers and questions

Solved Van der Waals equation is given below: P = ( nRT, )

6.3: Van der Waals and Other Gases - Physics LibreTexts
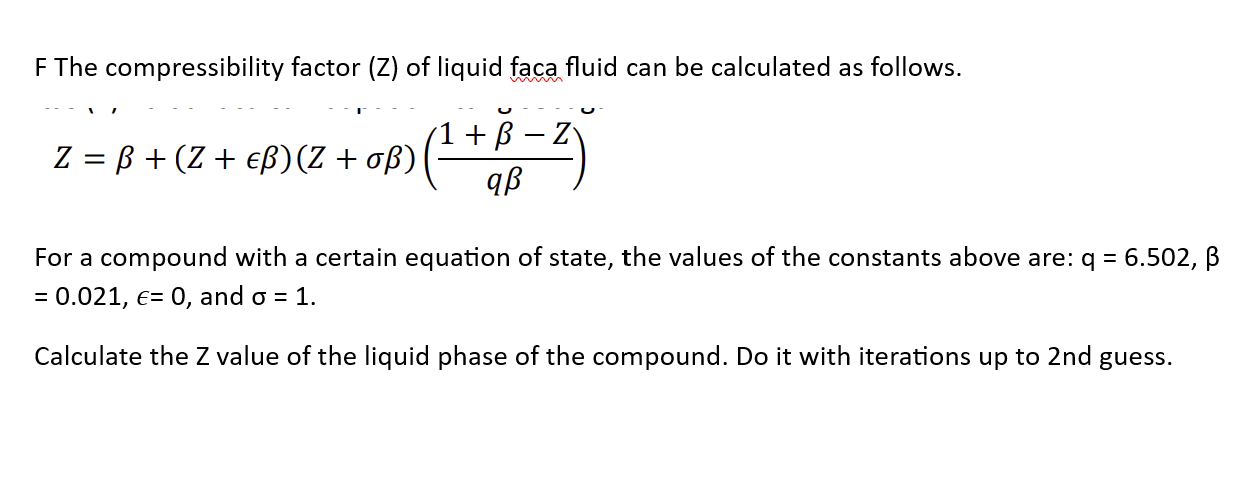
- Solved F The compressibility factor ( Z ) of liquid faca
- 3.2 Real gas and compressibility factor – Introduction to

- Compressibility factor (Z=(PV)/(nRT)) is plotted against pressure

- Compressibility factor Z for sub-critical pressures in a 'one-cell' formula for excel spreadsheets

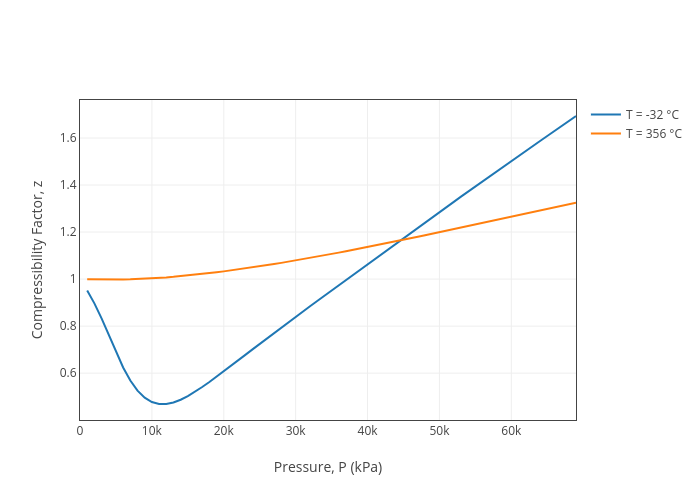
- Compressibility Factor, z vs Pressure, P (kPa), line chart made by Jdvani
- Ladies Size UK 10 12 EU 38 40 Lululemon

- Halloween trans flag Photographic Print for Sale by Ren Andrews

- High-Waist City Wise Cargo Pants in Dark Olive by Alo Yoga - Work Well Daily

- 8 Tricks to Make Wearing Black in Summer Chic and Comfortable - MY CHIC OBSESSION

- Odoreze® Natural Dumpster & Chute Odor Eliminator & Cleaner Concentrate