Compressibility factor Z = PV / nRT is plotted against pressure as

By A Mystery Man Writer
Compressibility factor Z = PV / nRT is plotted against pressure as shown below:What is the correct order for the liquefiability of the gases shown in the above graph? A. CO 2
Compressibility factor Z - PV - nRT is plotted against pressure as shown below-What is the correct order for the liquefiability of the gases shown in the above graph- A- CO 2- CH 4- N 2- H 2B- H 2- CH 4- N 2- CO 2C- CH 4- H 2- N 2- CO 2D- H 2- N 2- CH 4- CO 2
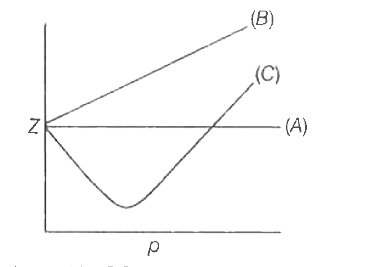
Telugu] The variation of compressibility factor (Z) with pressure (p

Gas Compressibility - an overview
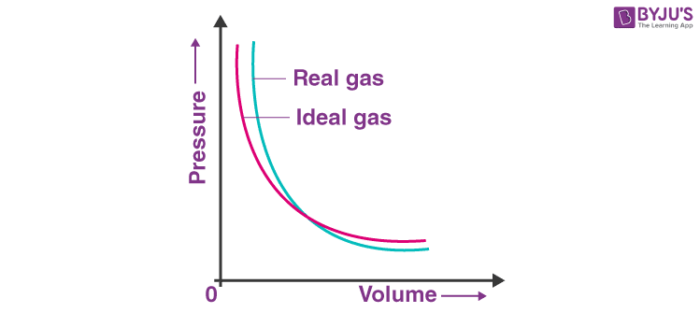
Deviation Of Real Gas From Ideal Gas Behavior

The graph of compressibility factor (Z) vs. P for one mole of a real gas is shown in following

The given graph represent the variations of Z (compressibility factor (Z)=dfrac {pV}{nRT}) versus P, three real gases A, B and C. Identify the only incorrect statement.For the gas B, b=0 and its

Compressibility factor - Wikipedia

Comparison of selected experimental compressibility factors (Z = P V

A real gas M behaves almost like an ideal gas. Graph 1 is obtained by plotting volume, V against temperature, T for x mol of gas M at pressure, P_1. a. Suggest

What is the compressibility factor? What is its value an ideal gas? How does it to understand the extent of deviation of a gas from ideal behavior?
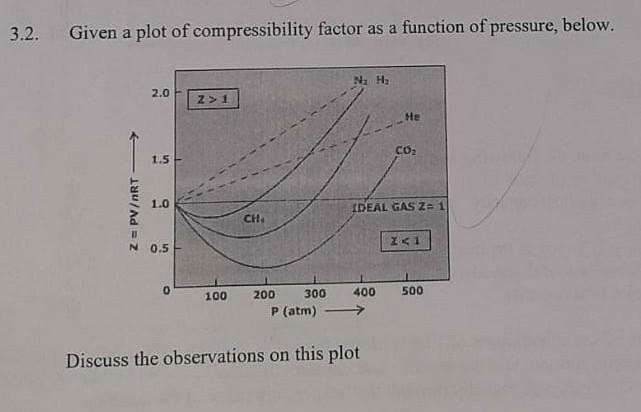
Solved 3.2. Given a plot of compressibility factor as a
What is the effect of pressure on real gas? - Quora

A real gas M behaves almost like an ideal gas. Graph 1 is obtained by plotting volume, V against temperature, T for x mol of gas M at pressure, P_1. a. Suggest







