Breaking local symmetry—why water freezes but silica forms a glass

By A Mystery Man Writer
Everyone knows that water freezes at 0 degrees C. Life on Earth would be vastly different if this were not so. However, water
Everyone knows that water freezes at 0 degrees C. Life on Earth would be vastly different if this were not so. However, water's cousin, silica, exhibits wayward behavior when cooled that has long puzzled scientists.
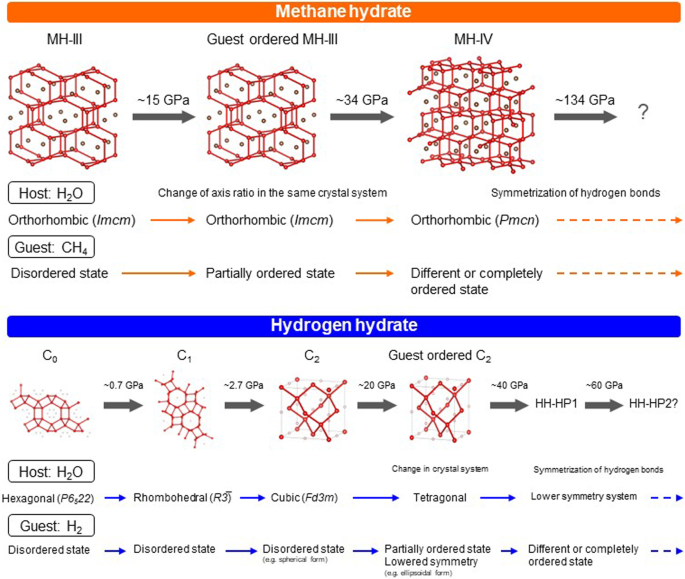
Significance of the high-pressure properties and structural evolution of gas hydrates for inferring the interior of icy bodies, Progress in Earth and Planetary Science

Schematic figure explaining the relationship between the behaviour of

Scientists offer designer 'big atoms' on demand

Breaking translational symmetry via polymer chain overcrowding in molecular bottlebrush crystallization
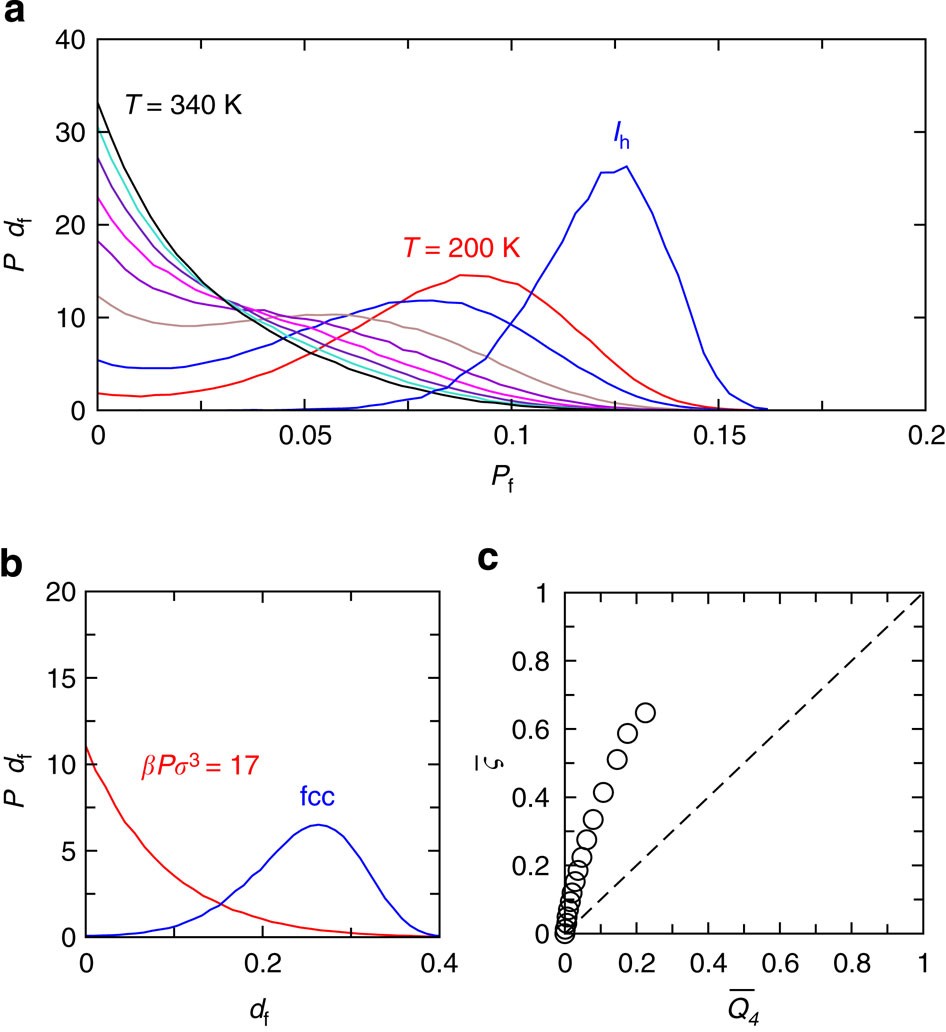
Understanding water's anomalies with locally favoured structures

Q&A: How Moving Water Freezes – SKY LIGHTS

Coatings, Free Full-Text
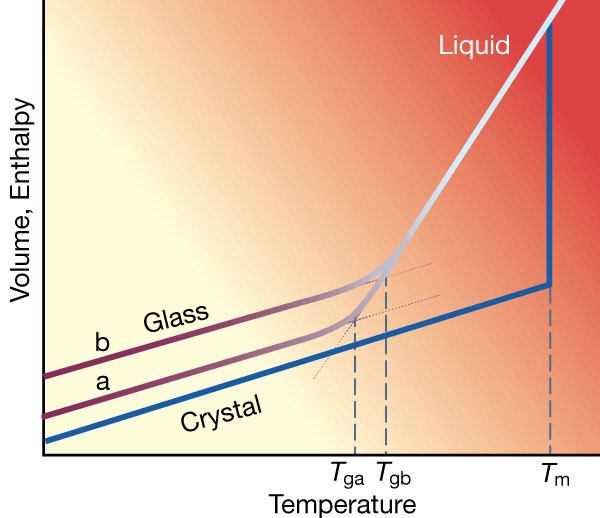
Supercooled liquids and the glass transition
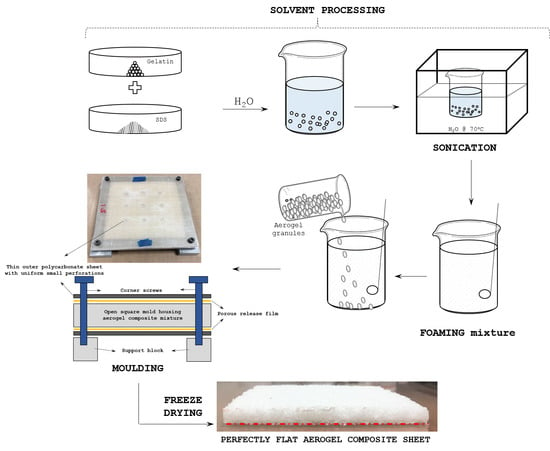
Gels, Free Full-Text
U Tokyo – sciencesprings

Is glass transition driven by thermodynamics?

Fast vs slow water—explaining the fragile-to-strong transition
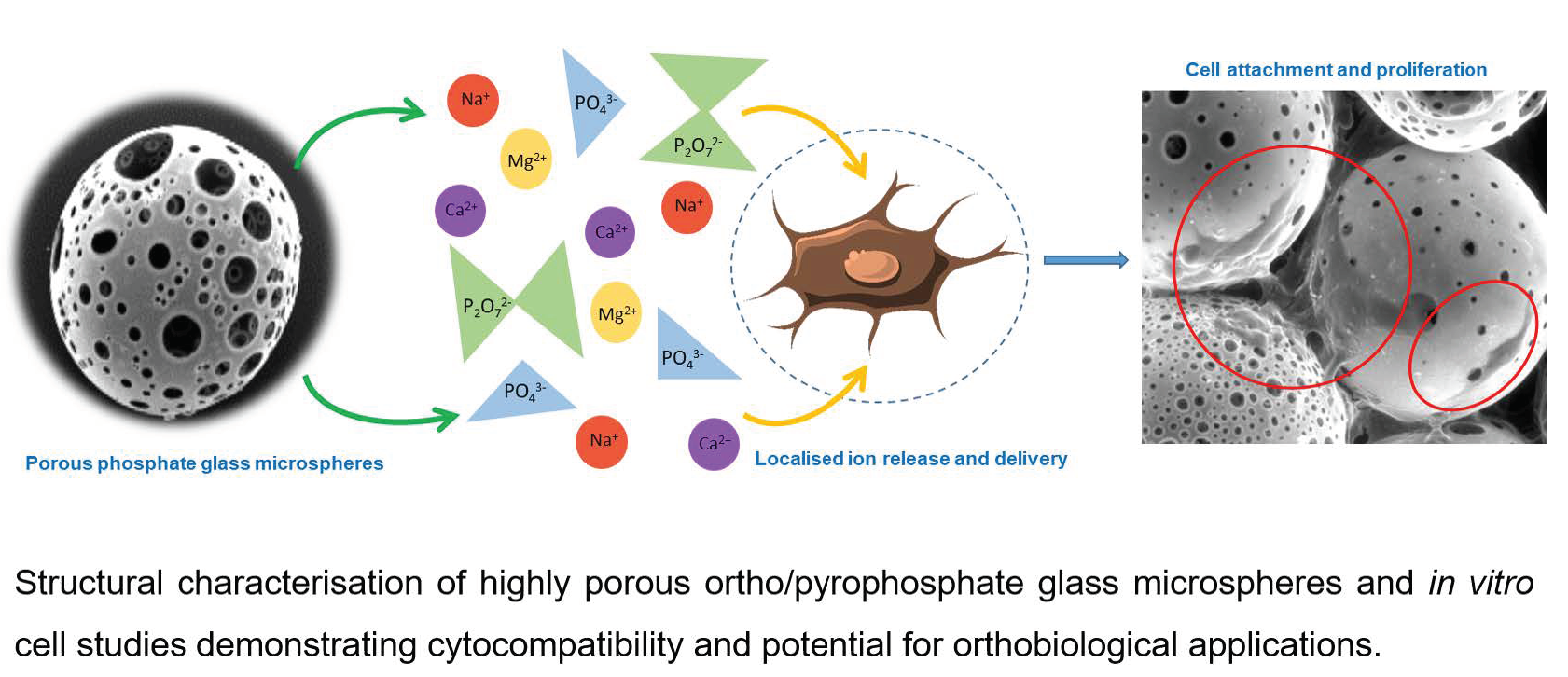
Bioengineering, Free Full-Text

Tetrahedrality is key to the uniqueness of water

Structure and dynamics of nanoconfined water and aqueous solutions
- Hippie Boho Wrap Top, Multi-way Scarf Top, Backless Halter Top, 70s Style Bohemian Top

- Fine Fragrance Collection - Happy International Millionaire Day
- 200 MARINA COLLECTION - Grey Stretch Jean, Distressed - Traditional Fit - Grey - - GRAND RIVER #200 Grey Stretch Traditional Fit Jean, GRANDRIVER Grey Stretch Jean, GRAND RIVER, GRANDRIVER, RIVER

- L. Organic Cotton Super and Super Plus Absorbency Compact Tampons 30 Count

- Bralettes for Women Padded Sports Bra Seamless Comfort Bra Wirefree Yoga Cami Tank Tops Bras for Womens, 1 Pack






