ISPE GAMP® 5: A Risk-Based Approach to Compliant GxP Computerized

By A Mystery Man Writer

GAMP 5 Second Edition – the Changes Pharmaceutical Laboratories Need to Know - Westbourne
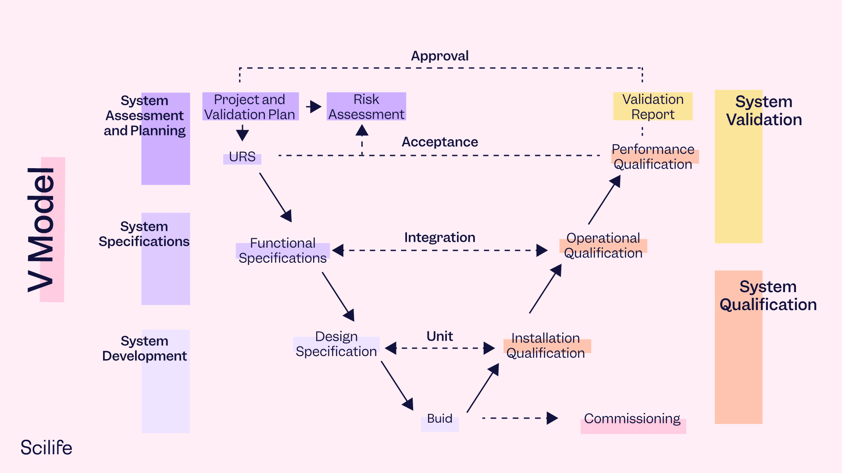
/hs-fs/hubfs/vmodel-v02.png?width=84

GAMP Good Practice Guide: GxP Compliant Laboratory Computerized Systems 2nd Edition, ISPE

ISPE GAMP5 Second Edition What's New?

PDF) Quality Risk Management for Computerised Systems-A Review

ISPE on LinkedIn: ISPE GAMP® 5: A Risk-Based Approach to Compliant GxP Computerized Systems…

Computerised Systems Validation - GAMP 5 Training Course

GAMP 5 Guide 2nd Edition, ISPE

GAMP 5 ®: How to take a risk-based approach to GP computerised systems

ISPE GAMP 5: A Risk-Based Approach to Compliant GxP Computerized Systems, Second Edition PDF - Technical Standards Store
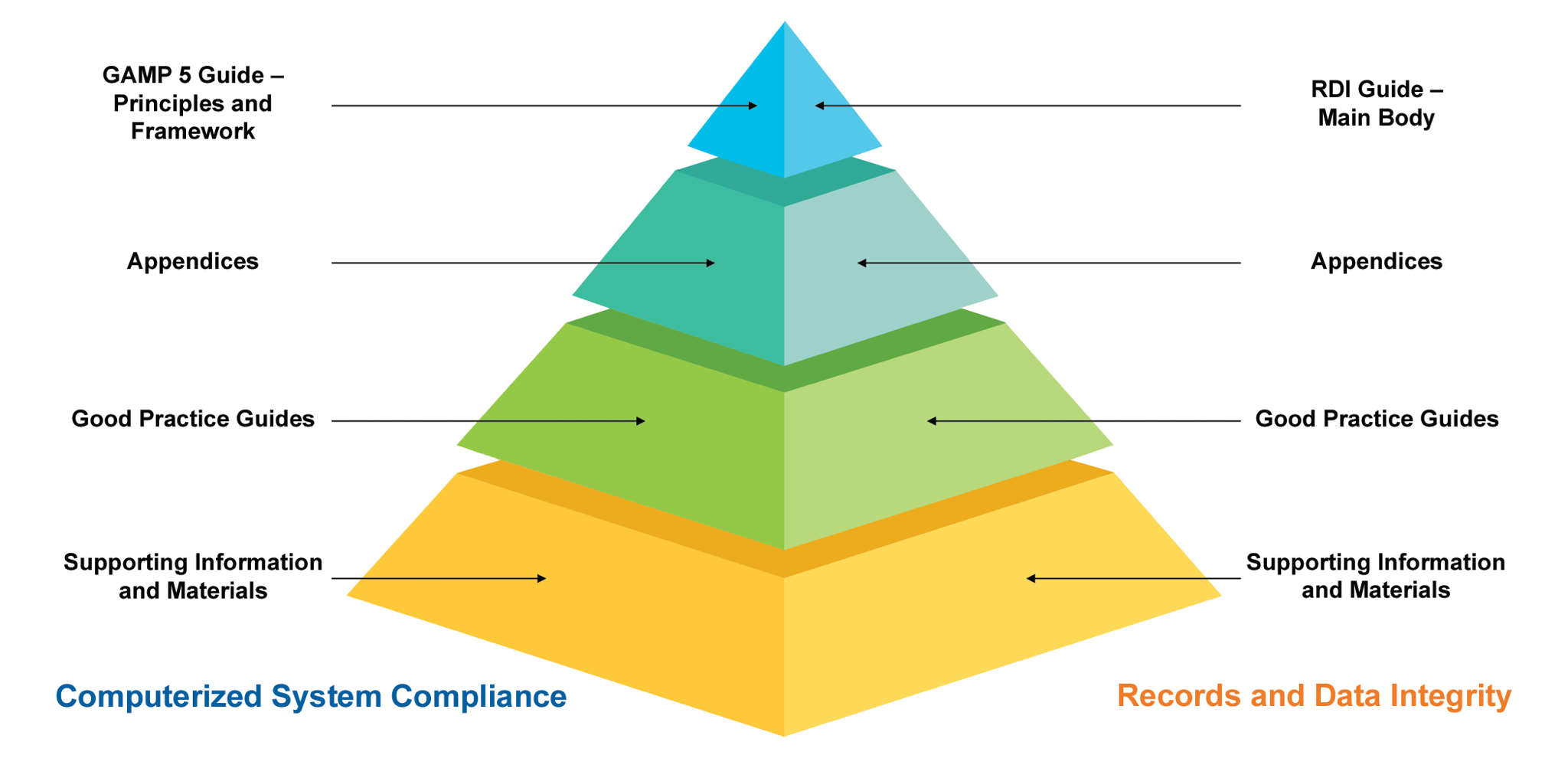
ISPE GAMP5 second edition: What's new?

CQV: Where to Start? A Comprehensive Guide to Commissioning, Qualification, and Validation

GAMP 5 for GxP Compliant Computerized Systems

Discover The Differences Of The GAMP®5 Second Edition, 58% OFF

GAMP Good Practice Guide: A Risk-Based Approach to GxP Compliant Laboratory Computerized Systems (Second Edition) – Most Currently Technical Standards
- Delimira Women's Seamless Bra Plus Size Smooth Full Figure Underwire Comfortable Minimizer Bras Brassiere - Bras - AliExpress

- Sleepover Pajamas for Girls Size 6-18 Dount cat Panama

- Cropped shot of a slim lady wearing a black bra and black and white lace erotic

- performance apparel Archives - Coffmans

- Buy ladies nikar fancy in India @ Limeroad





