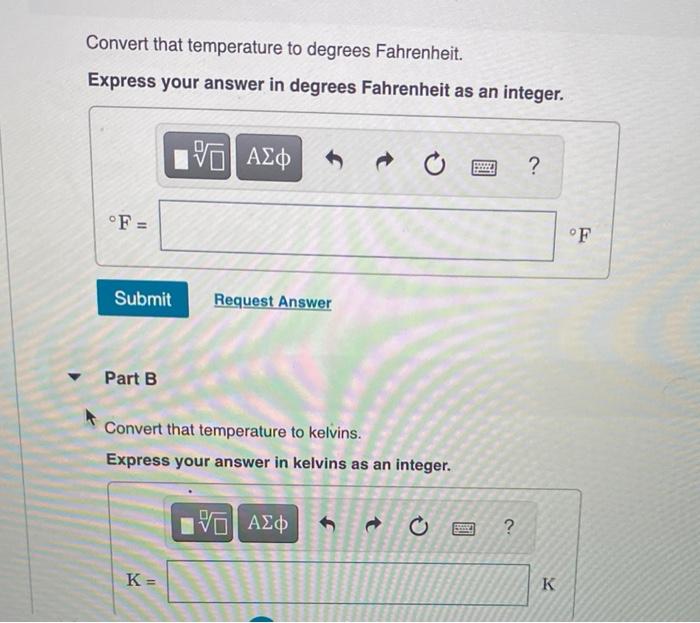
Solved Exercise 3.69 The freezing point of water is
By A Mystery Man Writer

The freezing point of a solution containing `50 cm^(3)` of ethylene glycol in `50 g` of water is

Solved Exercise 3.69 The freezing point of water is

SOLVED: Calculate the boiling point in degrees Celsius of a 3.69 m solution of KI in water. Remember that KI is an electrolyte. The normal boiling point of pure water is 100.0°C.
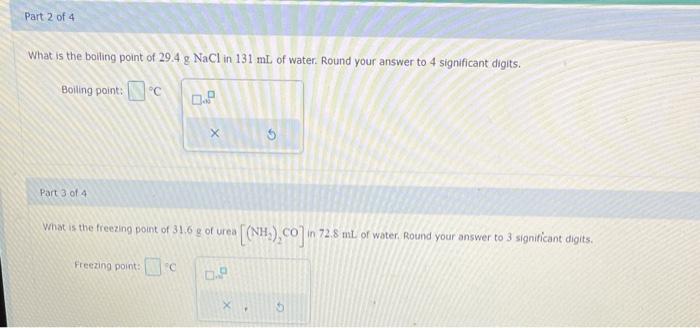
Solved What are the freezing points and boiling points of

Example of one water molecule between the two sodiums in the first peak

At home, you dissolve 26.0 g of AlCl3 in 1.5 kg of water. What is the freezing point of this solution?
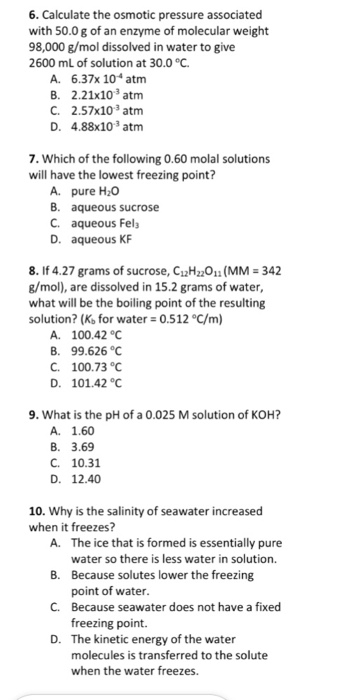
Solved 6. Calculate the osmotic pressure associated with

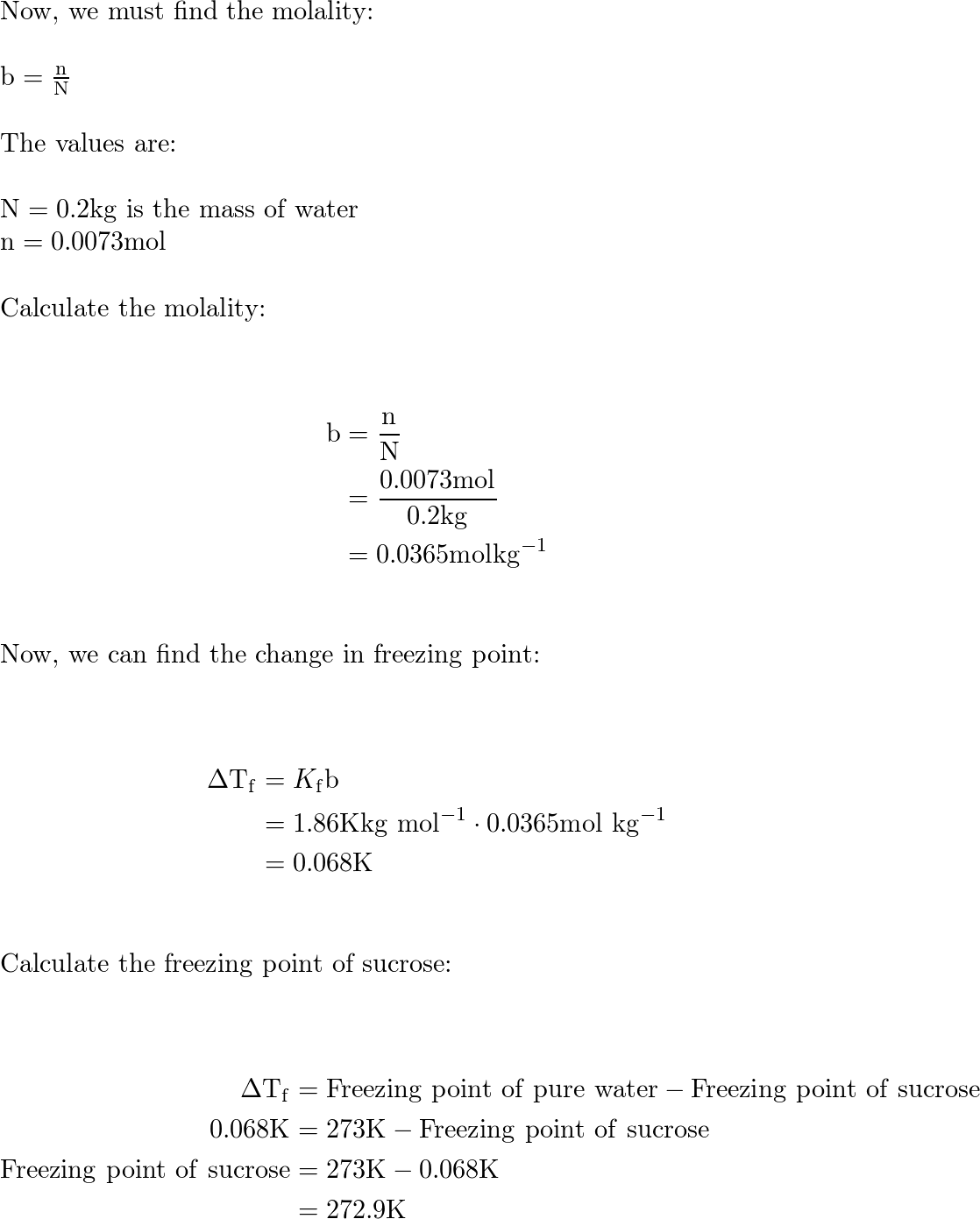
Pure water freezes at 273 K and 1 bar. The addition of 34.5 g of ethanol to 500 g of water changes the freezing point of the solution. Use the freezing point

SOLVED: Determine the freezing point depression of a solution that contains 30.7 g glycerin (C3H8O3, molar mass = 92.09 g/mol) in 500 mL of water. Assume the density of water is 1.0
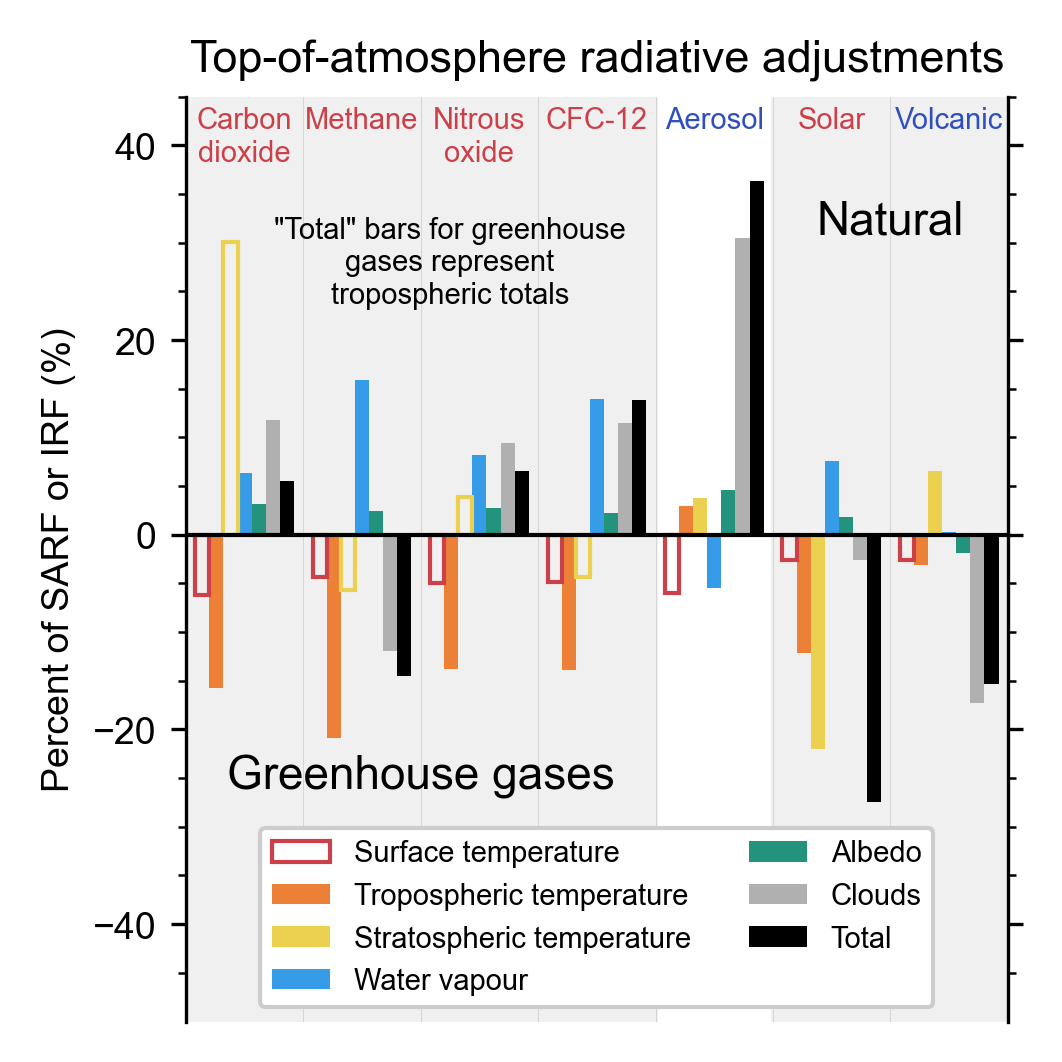
Chapter 7: The Earth's Energy Budget, Climate Feedbacks, and Climate Sensitivity
- SOLVED: what is 45 degree fahrenheit in celsius and kelvin. What
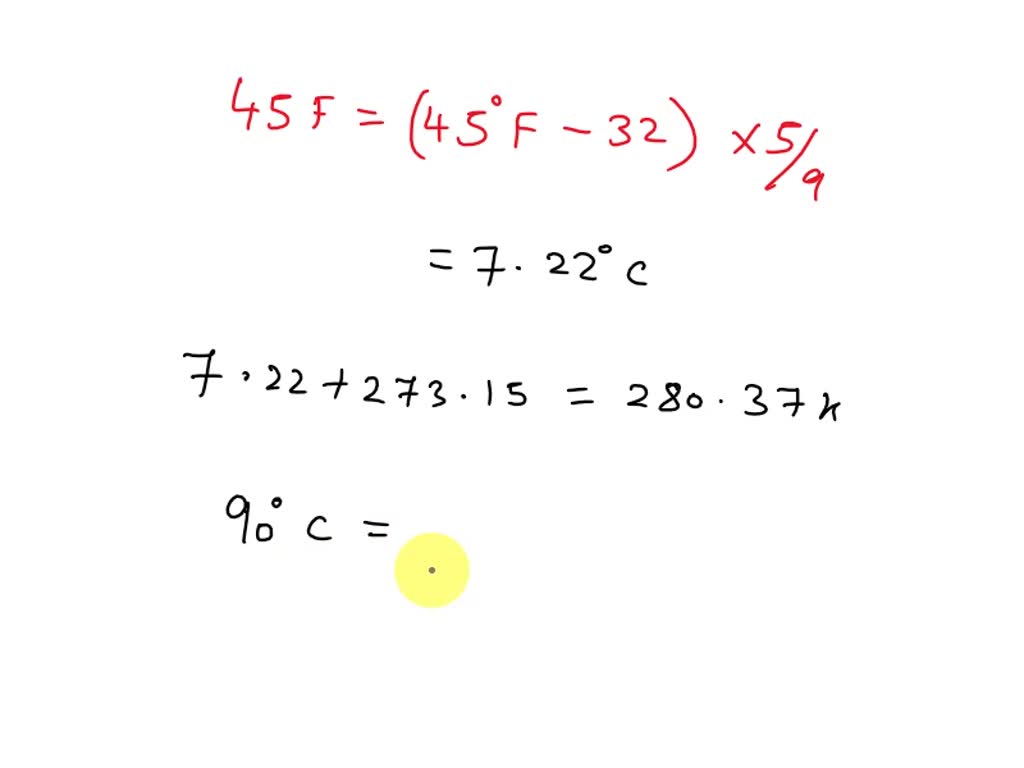
- USAF may convert some F-15Cs to radar jammers, News

- CONVERSION WORKSHEETS | TEMPERATURE

- On converting 45°C and 98.6°F to K, what will be the correct sequence of temperatures? (A) 318, 320 (B) 328,

- Heat Transfer NOTES. Thermal Energy TOTAL energy of motion in molecules of a substance (therm=heat) - ppt download





