4. A container contains 32 g of O2 at a temperature TThe pressure
By A Mystery Man Writer
4. A container contains 32 g of O2 at a temperature TThe pressure of the gas is P. An identical containercontaining 4 g of H2 at a temperature 2T has apressure of(1) 8P(3) P(2) 4P(4) P18r cnstant
4- A container contains 32 g of O2 at a temperature TThe pressure of the gas is P- An identical containercontaining 4 g of H2 at a temperature 2T has apressure of-1- 8P-3- P-2- 4P-4- P18r-cnstant

Answered: At what temperature Celsius will 19.4 g…

A container contains 32 g of O2 at a temperature T. The pressure of th

Maximum density of H,O is the temperature : (2) 39.2°F (1) 32°F

A container contains 32 g of O, a temperature T. The pressure of the gas is P. An identical container containing 4 g of H, a temperature 2T has a pressure of (

Solved 32. Which graph best shows the relationship between
A vessel has 6g of oxygen at a pressure P and temperature 400 k. A small hole is made in it so that O2 leaks out. How much O2 leaks out if

2.8 g of N2, 0.40 g of H2 and 6.4 g of O2 placed in a container of 1.0 L capacity 27°C. The total pressure in the container is : A) 6.15
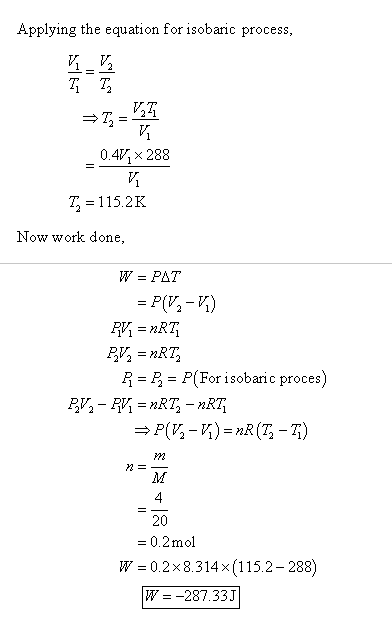
Answered: A container holds 4g of neon at a…

A container contains 32 g of O2 at a temperature T. The pressure of th

The Ideal Gas Law - Video Tutorials & Practice Problems

8.2: Relating Pressure, Volume, Amount, and Temperature: The Ideal Gas Law

Solved Question completion QUESTION 25 A container contains

A vessel contains 28 gm of N−2 and 32 gm of O2 temperature T = 1800 K and pressure 2 atm pressure it N2 dissociates 30 and O2 dissociates 50 temperature remains constant.

Solved e) 02- 32 (4 points) A glass vessel contains 16.0 g
- Fralda Babysec Ultrasec Mega G 32 Unidades - Softys - Mobile

- Great New Travel Softbox for Godox AD100, AD200, Speedlights, SMDV flip 32 G

- Chocolate Wafer Trento Diversos Sabores 32g - Peccin
- Fralda Descartável Babysec Galinha Pintadinha Ultra Mega Tamanho G

- Tinta de Choco 8 Unidades embalagem 32 g · Delfin · Supermercado

- Maidenform Casual Comfort Lace Bralette & Reviews

- Pilates pose #1 Tote Bag by Sasha Kircanski - Pixels

- AherBiu Women's Lingerie Sleep Lounge Bralette Crossover Back Yoga Bras Tops for Women Ribbed Wireless Wireless Bra

- 3 Hook Bra Extender - 3 Pack – Forever Yours Lingerie

- Is 'Women at War' (aka 'Les combattantes') on Netflix UK? Where to

- QUCO Brand Women Underwear Ice Silk Seamless Lace Briefs Sexy Lingerie Womens Panties 220426 From Long01, $13.97

- 13571-707 Thermal trousers - MASCOT® ORIGINALS

- Visiting a Long Neck Tribe, Thailand - All You Need to Know

- 8.800+ Loja De Esportes fotos de stock, imagens e fotos royalty

- Fashion Bling Gold Body Chains For Women Bulk Plus Size Rhinestone Breast Bondage Crystal Bra Chain Jewelry Underwear Top - Body Chain - AliExpress

