Solved Problem 3 (Phase changes) A 35 g ice cube at 0°C is

By A Mystery Man Writer
Answer to Solved Problem 3 (Phase changes) A 35 g ice cube at 0°C is

SOLVED: The specific heat capacity of ice is about 0.5 cal per gram per degree Celsius. Suppose it remains at that value all the way to absolute zero. Determine the quantity of
What temperature is ice made at? - Quora

14.3 Phase Change and Latent Heat

An ice cube having a mass of 50 grams and an initial temperature of -10 degrees Celsius is placed in 400 grams of 40 degrees Celsius water. What is the final temperature
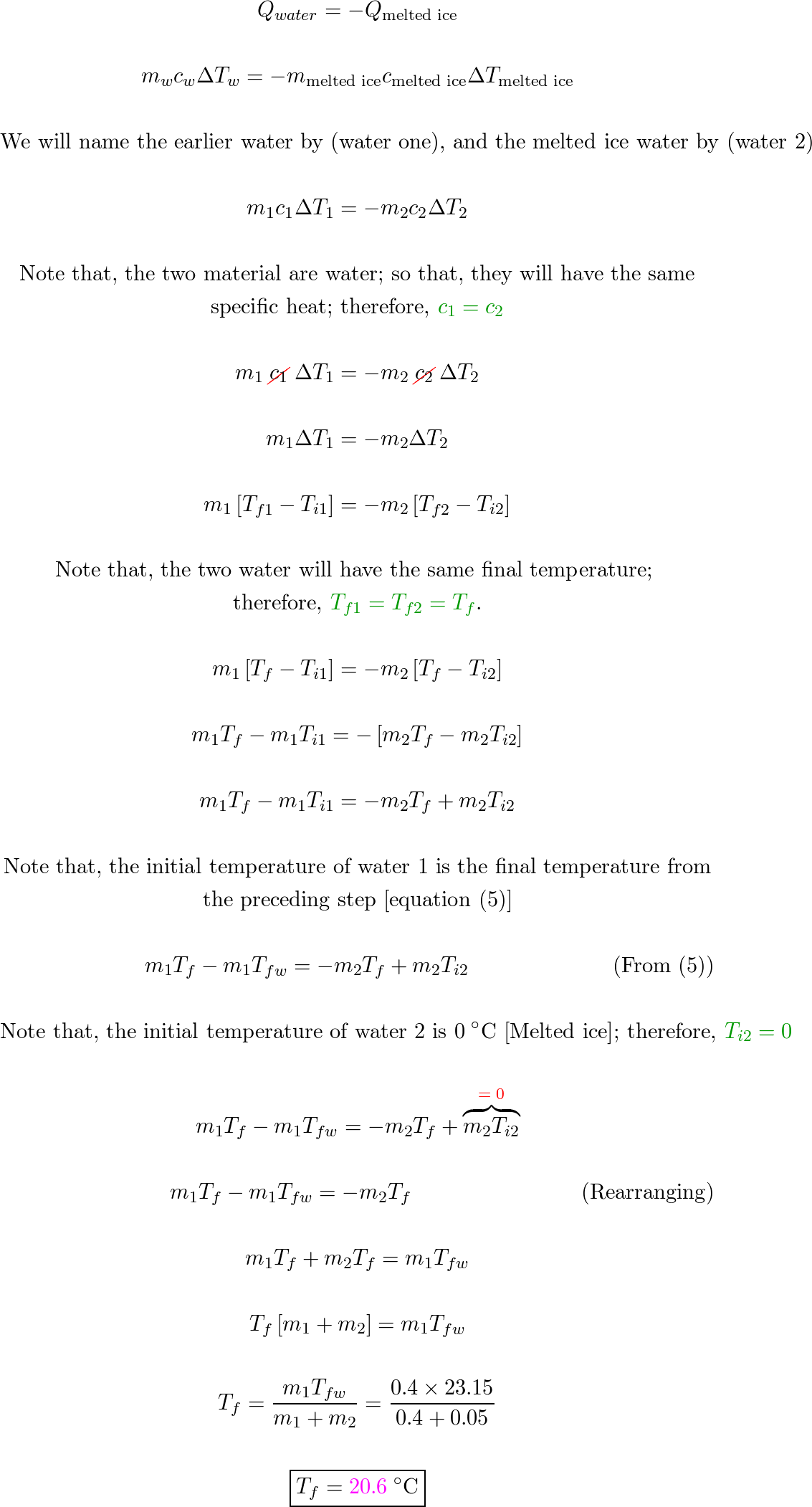
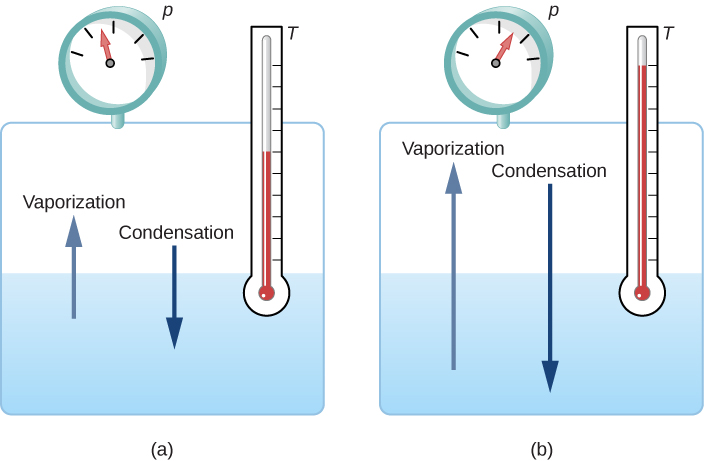
1.5 Phase Changes – University Physics Volume 2

14.3 Phase Change and Latent Heat – College Physics: OpenStax
What is a graph showing a phase change in temperature when ice is heated from -10C to over 100C? - Quora

Answered: The enthalpy of solution (∆ H) of NaNO3…

Solved A 53.3-g ice cube is initially at 0.0°C. (a) Find the
How much energy is required to change a 35-g ice cube from ice at -25 degrees C to steam at 115 degrees C? - Quora
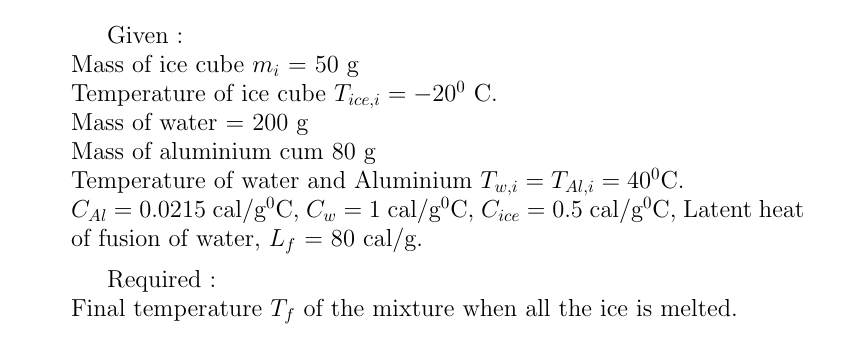
Answered: A 50 g ice cube, initially at -20degree…
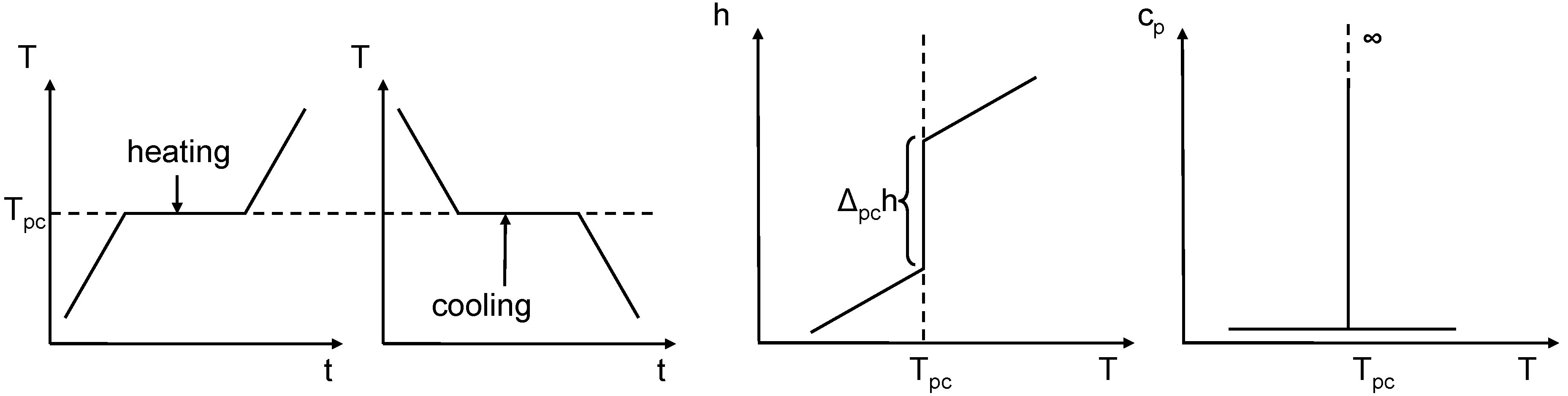
Applied Sciences, Free Full-Text
✓ Solved: A 50.-kg ice cube at 0°C is heated until 45 g has become water at 100.°C and 5.0 g has become
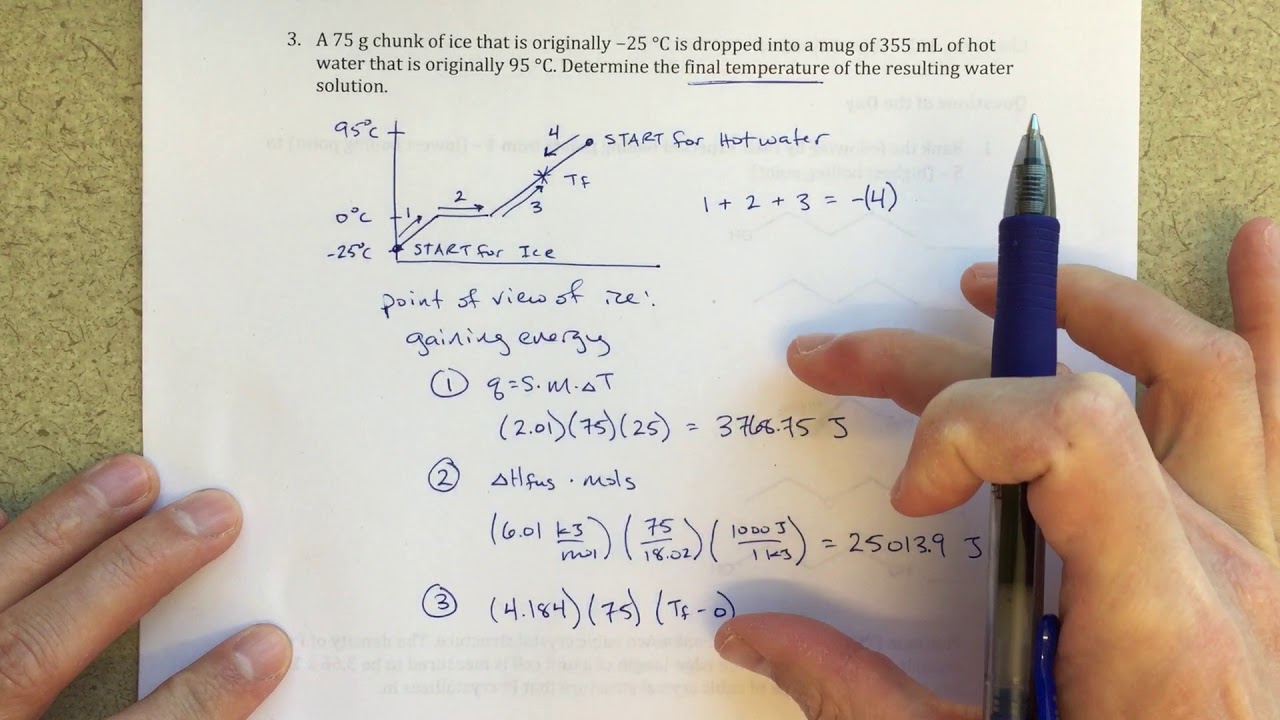
Finding Final Temperature When Ice is Added to Water
- 35 GRAMS 40 GRAMS 45 GRAMS 50 GRAMS 83 MM NECK PET PREFORM FOR JARS in Karaikal at best price by HN Plastics Pvt Ltd - Justdial

- 70ml 35g Plastic Measuring Spoon 35 Gram PP Measure Scoop for Pet

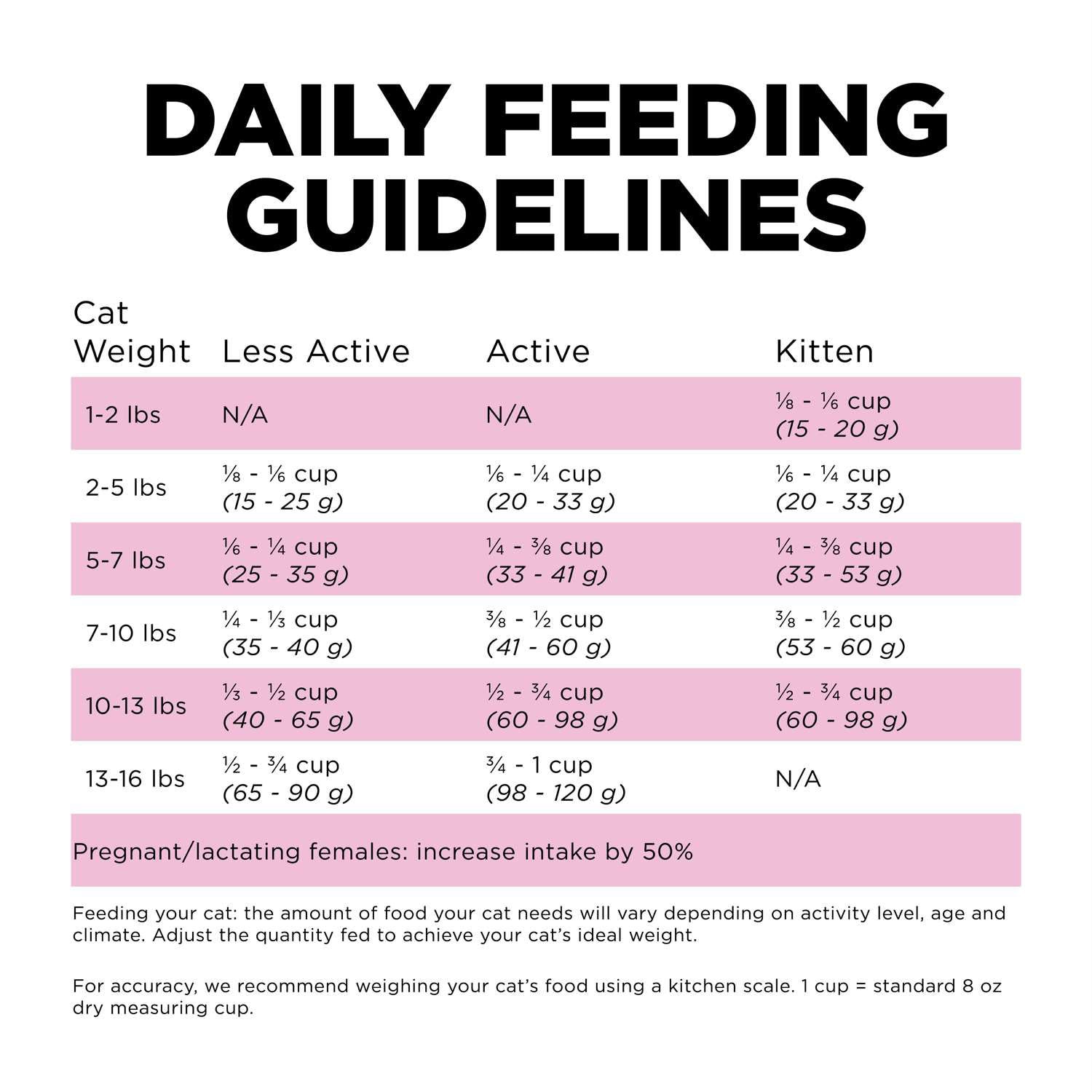
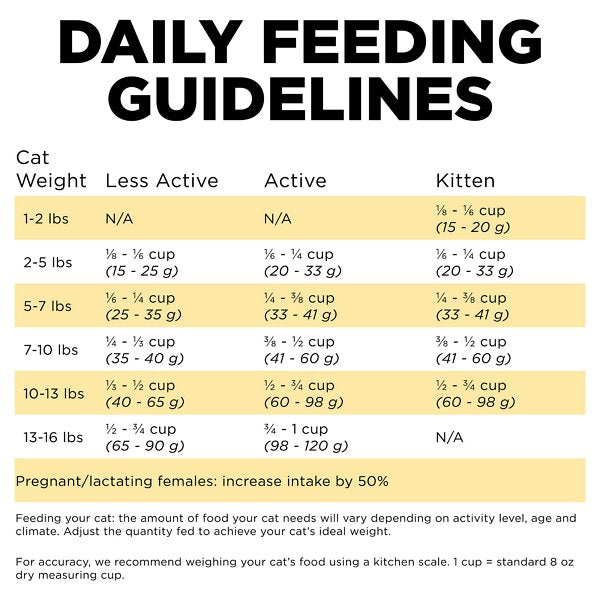
- GO! SOLUTIONS SKIN + COAT CARE Chicken Recipe Cat Food - Pet Valu
- Elite Black Ivory Coffee 6 packs - 35 grams each (30 cups of coffee)

- GO! SENSITIVITIES Limited Ingredient Grain Free Duck for cats – PetMax
- Zivame - #ContestAlert Calling All Brides-To-Be! Share A Picture Of You Bridal Attire In The Comments Below (Or DM Us). You Could Stand A Chance To Win A Gorgeous Bridal Hamper Along
- Saalt Soft Cup

- 866+ Panties PNGs: Royalty-Free Stock PNGs - PIXTA

- Open Road Brands Open Road Brands 90167605-S Railroad Crossing Prismatic Embossed Tin Sign 90167605-S

- Victoria's Secret, Intimates & Sleepwear, Victoria Secret Lace Balconette Bra 36c

